Nitrolingual Pumpspray
Druckfarbe: schwarz
Package leaflet: Information for the user
Nitrolinguaf Pump Spray
glyceryl trinitrate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist or nurse has told you.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
• You must talk to a doctor if you do not feel better.
What is in this leaflet:
1. What Nitrolingual® is and what it is used for
2. What you need to know before you take Nitrolingual®
3. How to take Nitrolingual®
4. Possible side effects
5. How to store Nitrolingual®
6. Contents of the pack and other information
1. What Nitrolingual® is and what it is used for
Nitrolingual® is a sublingual spray which means that you use it under your tongue. The active ingredient is called glyceryl trinitrate or GTN for short. GTN is one of a group of medicines called ‘nitrates’. These relax the muscles around the blood vessels and make it easier for the heart to do its work.
Nitrolingual® helps stop the pain of angina (pain in your chest, arms or neck especially when you exert yourself). You can also use the medicine immediately before doing things which you know will cause you angina pain.
2. What you need to know before you take Nitrolingual®
Do not take Nitrolingual®
• If you are allergic (hypersensitive) to nitrates or any of the other ingredients of Nitrolingual® (listed in Section 6). An allergic reaction may include rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
• If you are very ill because of very low blood pressure, severe blood loss, acute stroke, bleeding in the brain, a severe head injury or severe anaemia
• If you have certain unusual heart conditions (such as acute circulatory shock (where there is insufficient blood flow reaching the body’s tissues), this can include hypovolaemic shock (as a result of low blood volume) and uncontrolled cardiogenic shock (as a result of decreased output from the heart), severe mitral stenosis (a narrowing of the opening to the heart mitral valve) or obstructive cardiomyopathy (a disease of the heart muscle causing obstruction of blood flow)), which your doctor will have told you about
#

• If you are taking Viagra (sildenafil) or similar products (e.g., vardenafil, tadalafil) for the treatment of erectile dysfunction or hypertension of arterial lung vessels. If you take these products and Nitrolingual®, a severe and possibly dangerous fall in blood pressure can occur. This would result in collapse, unconsciousness and could be fatal.
Nitrolingual® Pump Spray is not intended for use in children.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking Nitrolingual®
• If you are in the early stages of an eye condition called glaucoma (where there is raised pressure within the eye)
• While taking Nitrolingual®, tell your doctor if the spray does not stop the pain; or if the spray usually works, but this time the pain lasts longer (half an hour or more), or feels different or worse than usual.
• if you have aortic and/or mitral stenosis (a narrowing of the opening to the heart aortic or mitral valve)
• if you feel dizzy when you sit or stand upright suddenly
• if you have cerebrovascular disease (brain disorders relating to disease of the blood vessels supplying the brain)
• If you have pericardial tamponade (compression of the heart caused by blood or fluid accumulation in the space between the heart muscle and the outer covering of the heart)
• if you have constrictive pericarditis (inflammation and swelling of the covering of the heart)
• low blood oxygen in lung disease or pulmonary heart disease (enlargement of the right ventricle of the heart)
• if you have had a heart attack
• If you have left ventricular hypertrophy (thickening of the muscle of the left ventricle of the heart) associated with aortic stenosis (narrowing of the opening of the aortic heart valve)
• if you have moderate to severe valvular aortic stenosis (narrowing of the opening of the aortic heart valve)
Other medicines and Nitrolingual®
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines. This is important as using more than one medicine at the same time can strengthen or weaken the effect of the medicines. Your doctor may need to take special care or change the dose. This is especially important for:
• medicines for the treatment of erectile dysfunction or hypertension of arterial lung vessels (see ‘Do not take Nitrolingual®’)

• other medicines which can lower blood pressure, such as beta-blockers, calcium channel blockers and neuroleptics, vasodilators, anti-hypertensives, diuretics, tricyclic antidepressants, sapropterin
• anti-blood-clotting drugs such as heparin
• N-acetyl-cysteine
If you use Nitrolingual® very often or if you regularly use other nitrates, the pain relief you receive may be less.
During use with dihydroergotamine (DHE) (used to treat migraines), Nitrolingual® may lead to an increase in DHE
levels, thereby increasing blood pressure.
Nitrolingual® with alcohol
If you drink alcohol before using Nitrolingual®, you may feel dizzy or faint due to low blood pressure.
Pregnancy, breast-feeding and fertility
Tell your doctor if you become pregnant while you are taking Nitrolingual®. You should use Nitrolingual® only after
discussing with your doctor the potential benefits to you versus any potential risks to your unborn child. It is not known whether glyceryl trinitrate passes into human breast milk. You should ask your doctor for advice if you are breast-feeding. There is no sign of a harmful effect with respect to fertility.
Driving and using machines
You should wait at least five minutes after using the spray before driving or using machinery. If you feel faint, dizzy or unwell, wait until you feel better. You should be particularly careful if you have just started using Nitrolingual®, if you have changed your dosage or if you drink alcohol.
220420025/12 Druckdatum
220420025/12 Gl Nitrolingual Pump Spray / 150 x 260 mm / LC 7939 / England (tub)
220420025-12_3K_01-16.indd 1
27.01.16 09:15
Druckfarbe: schwarz
Nitrolingual® contains ethanol
This medicinal product contains small amounts of ethanol (alcohol); less than 10 mg per metered dose (puff).
3. How to take Nitrolingual®
Dosage
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure. The spray is meant for use under your tongue and is not meant to be inhaled.
The recommended dose is one or two puffs under your tongue. If symptoms do not resolve, you can repeat this at 5 minute intervals for a maximum of two more times, for a total of three doses. If, after that, your symptoms have still not resolved, please seek immediate medical attention. The spray should work quickly and last about half an hour. Method of administration
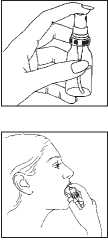
Before you use a new bottle of Nitrolingual®, spray the first puff into the air to get the pump working properly. You must also do this if you have not used the pump for a week or more. Get used to the feel of the grooved button in case you need to use the pump in the dark.
1. Rest or sit quietly, as you may feel faint or dizzy otherwise, particularly if you are elderly.
2. Hold the bottle upright with your finger on the button. You don’t need to shake the bottle.
3. Open your mouth and put the bottle next to your chin (see picture).
4. Press the button firmly so that the puff of medicine goes under your tongue (see picture).
Close your mouth.
5. Do not breathe in while you are taking the puff of medicine.
Keep the spray with you at all times. Through the side of the bottle you can see how much spray you have left. Make sure that you get a new spray before the old one runs out. Always keep a
spare.
Talk with your doctor about how long you should keep taking Nitrolingual®.
If you take more Nitrolingual® than you should If you take too many puffs you may notice more severe and pronounced side effects (see section 4), for example, you may get a bad headache, blurred vision, feel hushed or feel that your heart is beating more slowly. You may also feel faint, sweaty, breathless, weak, restless and feel sick or be sick, or notice a bluish tinge to your lips or a bluish colouration of the skin. In very rare cases you may develop methaemoglobinaemia (a disorder of the red blood cells). If any of these effects persist contact your doctor or pharmacist.
If you forget to take Nitrolingual®
Do not take a double dose to make up for a forgotten dose.
If you stop taking Nitrolingual®
Do not stop taking Nitrolingual® without the advice of your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency of side effects is classified into the following categories:
|
Very common: |
may affect more than 1 in 10 people |
|
Common: |
may affect up to 1 in 10 people |
|
Uncommon: |
may affect up to 1 in 100 people |
|
Rare: |
may affect up to 1 in 1,000 people |
|
Very rare: |
may affect up to 1 in 10,000 people |
|
Not known: |
frequency cannot be estimated from the available data |
The following side effects have been reported:
Very common: headache
Common: decreased blood pressure, which can also occur on standing up; weakness; dizziness; drowsiness; increased heart rate
Uncommon: fainting; worsened angina symptoms; slowing of the heart rate; bluish colouration of the skin; facial
hushing; circulatory collapse (failure of the blood circulation); nausea; vomiting; allergic skin rash; hypersensitivity.
Very rare: cerebral ischaemia (decreased blood how to the brain); methaemoglobinaemia (a disorder of the red blood
cells); restlessness; difficulty breathing; skin rash
Not known: tongue swelling (due to an allergic reaction); tongue blistering
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Nitrolingual®
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and bottle after ‘EXP’. The expiry date refers to the last day of that month. Do not store Nitrolingual® above 25 °C. Do not throw away any medicines via wastewater or household waste. Ask your
pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Nitrolingual® contains
The active substance is GTN. Each puff of spray contains 400 micrograms of GTN.
The other excipients are fractionated coconut oil, ethanol (absolute), glycerol monocaprylocaprate and peppermint oil. What Nitrolingual® looks like and contents of the pack Red plastic coated glass bottle fitted with metering pump.
Each bottle contains 4.9 g, 10.3 g, 11.2 g or 14.2 g solution (equivalent to about 75,180,200 or 250 doses).
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Merck Serono Ltd Bedfont Cross, Stanwell Road Feltham, Middlesex, TW14 8NX, UK Manufacturer:
G. Pohl-Boskamp GmbH & Co. KG, Kieler Strasse 11,25551 Hohenlockstedt, Germany
This leaflet was last revised in January 2016
TW1018355
220420025/12


220420025-12_3K_01-16.indd 2 # 27.01.16 09:15