Noqdirna 25 Microgram Oral Lyophilisate
4. Carefully take a tablet out of its blister. Place the tablet under the tongue and allow it to dissolve. Do not chew or swallow the tablet.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Noqdirna'
25 micrograms oral lyophilisate 50 micrograms oral lyophilisate
Desmopressin
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Noqdirna is and what it is used for
2. What you need to know before you take Noqdirna
3. How to take Noqdirna
4. Possible side effects
5. How to store Noqdirna
6. Contents of the pack and other information
1. What Noqdirna is and what it is used for
Noqdirna contains desmopressin, an antidiuretic, which reduces urine production.
Noqdirna is used for the treatment of nocturia (frequent need to get up to urinate at night) due to nocturnal polyuria (overproduction of urine during night) in adults.
2. What you need to know before you take Noqdirna
Do not take Noqdirna:
- if you are allergic to desmopressin or any of the other ingredients of this medicine (listed in section 6)
- if you suffer from polydipsia (excessive thirst and increased fluid intake) or psychogenic polydipsia (psychologically caused increased thirst and increased fluid intake)
- if you have known or suspected cardiac insufficiency (heart failure in which the heart is not able to pump enough blood throughout the body)
- if you have any disease requiring treatment with diuretics
- if you have moderately or severely reduced kidney function
- if you have or have had hyponatraemia (low sodium level in the blood)
- if you have SIADH (hormone secretion disorder)
Warnings and precautions
Talk to your doctor or pharmacist before taking Noqdirna.
It is especially important that you talk to your doctor before taking Noqdirna if:
- you have severe bladder dysfunction and problems urinating
- you are 65 years or older since your doctor will have to monitor the level of sodium in your blood (see the section 3 “How to take Noqdirna” below)
- you have low levels of sodium in your blood
- you have a medical condition(s) causing fluid and/or electrolyte imbalance
- you have a medical condition(s) that could be made worse by fluid and/or electrolyte disturbance
- you get an acute intercurrent illness (such as systemic infection, fever and stomach flu) as it may be necessary for the doctor to interrupt/ reassess the treatment with Noqdirna
- you have cystic fibrosis, coronary heart disease, high blood pressure, chronic kidney disease or pre-eclampsia
You must limit fluid intake to a minimum from 1 hour before taking Noqdirna until 8 hours after taking Noqdirna. Treatment without simultaneous reduction of fluid intake may lead to water retention and/ or mineral imbalances with or without accompanying warning signs and symptoms hereunder such as headache, nausea/vomiting, weight gain and, in severe cases, convulsions.
Other medicines and Noqdirna
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
It is especially important you tell your doctor if you are taking:
- tricyclic antidepressants, which are medicines used to treat e.g. depression (such as clomipramine, imipramine, desipramine)
- selective serotonine reuptake inhibitors (SSRIs), which are medicines used to treat e.g. depression or anxiety (such as citalopram, paroxetine, sertraline)
- chlorpromazine, which is an anti-psychotic medicinal product used to treat e.g. schizophrenia
- diuretics (water tablets such as thiazides or other types of diuretics)
- carbamazepine, which is used to treat e.g. bipolar disorder and epilepsy
- antidiabetic medicinal products used for type II diabetes (medicines in the sulfonylurea group), particularly chlorpropamide
- non-steroidal anti-inflammatory drugs (NSAIDs), which are medicinal products used for the treatment of pain and inflammation (e.g. aspirin and ibuprofen)
- oxytocin, which is a medicinal product used around childbirth
- lithium, which is used to treat e.g. bipolar disorder
- Loperamide, which is a medicinal product used for the treatment of diarrhoea
Noqdirna with food and drink
Noqdirna should not be taken with food, since the effect may be reduced.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Your doctor will decide if you can use this medicine during pregnancy or if you are breast-feeding.
Driving and using machines
Noqdirna has no or negligible influence on the ability to drive and use machines.
3. How to take Noqdirna
Always take this medicine exactly as your doctor has told you.
Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
- Women: 25 micrograms daily, one hour before bedtime, administered under the tongue without water.
- Men: 50 micrograms daily, one hour before bedtime, administered under the tongue without water.
Noqdirna is placed under the tongue where it dissolves without the need for water.
Instructions for use
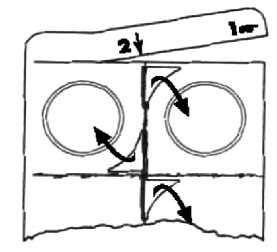
1. Completely remove the end tab of a blister strip by tearing along the perforations, starting from the corner with the hand symbol.
2. Now remove one blister from the strip by tearing along the perforations.
3. Remove the foil on each blister, starting at the corner with the printed arrow, by peeling off the foil in the direction of the arrow.
Do not push the tablet through the foil.
5. If a tablet breaks into more than two pieces while you are taking it out of its blister, do not take the broken pieces. Take a tablet from another blister.
You must limit fluid intake to a minimum from 1 hour before taking Noqdirna until 8 hours after taking Noqdirna. If you experience any of the following symptoms the treatment should be stopped and your doctor contacted: headache, nausea/vomiting, weight gain and, in severe cases, convulsions (see section 2 “Warnings and precautions”). Your doctor can choose to restart treatment. When restarting treatment, you must strictly restrict fluid intake. In addition, your doctor will closely monitor the sodium levels in your blood.
Use in elderly patients (65 years of age and older)
If you are 65 years or older your doctor will have to monitor the level of sodium in your blood before starting the treatment, during the first week of treatment (4-8 days after initiation of the treatment) and again in about one month after the initiation of the treatment.
Kidney impairment
If you have moderately or severely reduced kidney function, do not take Noqdirna. Talk to your doctor.
Liver impairment
If you have impaired liver function you should talk to your doctor before taking Noqdirna.
Use in children and adolescents
This medicine is for use in adults only.
If you take more Noqdirna than you should
It is important that you do not take more than the prescribed dose in any 24 hour period. Special attention should be given to signs of hyperhydration of the body (water intoxication), such as weight gain, headache, nausea and, in severe cases, convulsions.
Please consult your doctor if you have taken more Noqdirna than you should.
If you forget to take Noqdirna
Do not take a double dose to make up for a forgotten tablet.
Continue taking the tablets as usual on the next day.
If you stop taking Noqdirna
Treatment should only be interrupted or stopped on advice of your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Drinking too much fluid may lead to a build up of water which dilutes the salt in the body in severe cases. This can become a serious problem and may lead to convulsions.
STOP taking this medicine and tell your doctor immediately or go to your nearest casualty department if you experience one or more of these symptoms,
- an unusually bad or prolonged headache,
- confusion,
- unexplained weight gain,
- nausea or vomiting.
Side Effects include:
Very common: may affect more than 1 in 10 people
- Dry mouth
Common: may affect up to 1 in 10 people
- Nausea, feeling unwell, muscle weakness and confusion due to decreased level of sodium in the blood (hyponatraemia)
- Headache
- Dizziness
- Nausea
- Diarrhoea
Uncommon: may affect up to 1 to 100 people
- Constipation
- Stomach discomfort
- Weakness (fatigue)
- Swelling of the tissue in the lower limbs (peripheral oedema)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse.
This includes any possible side effects not listed in this leaet. You can also report side effects directly via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Noqdirna
Keep this medicine out of the sight and reach of children.
This medicinal product does not require any special temperature storage conditions. Store in the original package in order to protect from moisture and light. Use immediately upon opening individual tablet blister.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Noqdirna contains
- The active substance is desmopressin added as desmopressin acetate. Each oral lyophilisate contains 25 micrograms or 50 micrograms desmopressin.
- The other ingredients are gelatin, mannitol (E 421) and citric acid, anhydrous.
What Noqdirna looks like and contents of the pack
Noqdirna 25 micrograms:
White, round, oral lyophilisate tablet of approximately 12 mm marked with 25 on one side.
Noqdirna 50 micrograms:
White, round, oral lyophilisate tablet of approximately 12 mm marked with 50 on one side.
Laminated aluminium blister sheets in an outer carton. Each perforated unit dose blister sheet contains 10 oral lyophilisates
Pack size:
10, 30, 90 or 100 oral lyophilisates.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Ferring Pharmaceuticals Ltd.,
Drayton Hall, Church Road, West Drayton, UB7 7PS, UK.
Noqdirna 25 micrograms oral lyophilisate - PL 03194/0118 Noqdirna 50 micrograms oral lyophilisate - PL 03194/0119
Manufacturer:
Ferring GmbH, Wittland 11, D-24109, Kiel, Germany.
This medicinal product is authorised in the Member States of the EEA under the following names:
Nocdurna: Austria, Belgium, Cyprus, Czech Republic, Denmark, Germany, Greece, Finland, France, Croatia, Hungary, Iceland, Liechtenstein, Luxemburg, Malta, Netherlands, Norway, Portugal, Romania, Slovenia, Slovakia, Sweden. Noqturina: Estonia, Ireland, Latvia, Lithuania, Poland.
Noqdirna: UK.
HoKgypHa: Bulgaria.
This leaflet was last revised in April 2016.
Noqdirna, FERRING and the FERRING Logo are trademarks of Ferring B.V.
FERRING
PHARMACEUTICALS