Octanate 50 Iu/Ml Powder And Solvent For Solution For Injection
|
Octanate® powder vial size (IU FVIII) |
Solvent vial size (to be added to Octanate® powder vial) (ml) |
Nominal concentration of reconstituted solution (IU FVIII/ml) |
|
250 IU |
5 |
50 |
|
500 IU |
10 |
50 |
|
1000 IU |
10 |
100 |
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any side effects not listed in this leaflet.
5. How to store Octanate
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the vials in the outer carton in order to protect from light.
Use the reconstituted solution immediately and on one occasion only.
Do not use this medicine if you notice cloudy or incompletely dissolved solutions.
Any unused product or waste material should be disposed of in accordance with local reguirements.
6. Contents of the pack and other information
What Octanate contains
The active substance is the human blood coagulation factor VIII.
Volume and concentrations
The other ingredients are:
For the powder: sodium citrate, sodium chloride, calcium chloride, glycine For the solvent: water for injections.
What Octanate looks like and contents of the pack
Octanate is presented as a powder and solvent for solution for injection.
The powder is white or pale yellow, also appearing as a friable solid.
The solvent is a clear, colourless liguid.
The three available pack sizes differ in the amount of human blood coagulation factor VIII and solvent:
• 250 lU/vial: reconstitution with 5 ml leads to 50 lU/ml
• 500 lU/vial: reconstitution with 10 ml leads to 50 lU/ml
• 1000 lU/vial: reconstitution with 10 ml leads to 100 lU/ml
All pack sizes include:
one disposable syringe, one transfer set Mix2Vial™, one injection needle, and two alcohol swabs Not all pack sizes may be marketed.
Marketing Authorisation Holder
Octapharma Limited The Zenith Building 25 Spring Gardens Manchester M2 1AB United Kingdom
For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder.
Manufacturer
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Str. 235 A-1100 Vienna Austria or
Octapharma S.A.S
70 - 72 Rue du Marechal Foch
BP 33, F- 57381 Lingolsheim
France
or
Octapharma AB SE 1 12 75 Stockholm Sweden
This medicinal product is authorised in the member states of the EEA under the following names:
• Octanate: Austria, Belgium, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, The Netherlands, Malta, Norway, Poland, Portugal, Republic of Slovenia, Romania, Slovak Republic, Spain, Sweden, The United Kingdom
• Octafil: Finland
This leaflet was last revised in September 2012

Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Please keep this leaflet. You may need to read it again.
• If you have further questions, please ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
Package leaflet: Information for the user
Octanate 50 lU/ml Powder and Solvent for Solution for Injection
Human Coagulation Factor VIII
Octanate 100 lU/ml Powder and Solvent for Solution for Injection Human Coagulation Factor VIII
What is in this leaflet
1. What Octanate is and what it is used for
2. What do you need to know before you use Octanate
3. How to use Octanate
4. Possible side effects
5. How to store Octanate
6. Contents of the pack and other information
1. What Octanate is and what it is used for
Octanate belongs to a group of medicines called clotting factors and contains human blood coagulation factor VIII. This is a special protein involved in blood clotting.
Octanate is used to treat and prevent bleeding in patients with haemophilia A. This is a condition in which bleeding can go on for longer than expected. It is due to an hereditary lack of coagulation factor VIII in the blood.
2. What do you need to know before you use Octanate
• It is strongly recommended that every time you receive a dose of Octanate the name and batch number of the product are recorded in order to maintain a record of the batches used.
• Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly or repeatedly receive human-derived Factor VIII prodcuts.
Do not use Octanate
• if you are allergic to human blood coagulation factor VIII or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Octanate.
• Octanate contains very small amounts of other human proteins. Any medicine which contains proteins and which is injected into a vein (administered intravenously) can cause allergic
B.120.007.UK
* 1" *— reactions (See Section 4., "Possible side effects").
• Individuals with haemophilia A can develop factor VIII inhibitors (neutralising antibodies) (See Section 4., "Possible side effects").
Information about the blood and plasma used for Octanate
• When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients. These include careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded, and the testing of each donation and pools of plasma for signs of virus/infections. Manufacturers of these products also include steps in the processing of the blood or plasma that can inactivate or remove viruses. Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B (HBV) virus and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV). The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19.
Parvovirus B19 infection may be serious for pregnant women (infection of the baby) and for individuals whose immune system is depressed or who have some types of anaemia (e.g. sickle cell disease or abnormal breakdown of red blood cells).
Other medicines and Octanate
• Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without prescription.
• Human blood coagulation factor VIII products are not known to interact with other medicinal products. Nonetheless, do not combine Octanate with other medicines during infusion.
Pregnancy and breast-feeding
• Ask your doctor or pharmacist for advice before using any medicine.
Driving and using machines
• No effects on ability to drive and use machines have been observed.
Octanate contains
less than 1 mmol sodium (23 mg) per dose, i.e. essentially 'sodium-free' for 250 lll/vial and contains up to 1.75 mmol sodium (40 mg) per dose for 500 and 1000 lll/vial.To betaken into consideration by patients on a controlled sodium diet.
3. How to use Octanate
Octanate should be administered intravenously after reconstitution with the supplied solvent.
Treatment should be started under medical supervision.
Required units = body weight (kg) x desired increase in factor VIII (%) (lU/dl) x 0.5
|
Degree of bleeding/ Type of surgical procedure |
Factor VIII level required (%) (lU/dl) |
Frequency of dosage (hours between dosages) / Duration of therapy (in days) |
|
Bleeding | ||
|
Bleeding into a joint (early haemarthrosis), muscle bleeding or oral bleeding |
20-40 |
Repeat every 12 to 24 hours for at least 1 day, until the pain decreases or healing is achieved. |
|
More extensive bleeding into a joint (haemarthrosis), muscle bleeding or effusion of blood (haematoma) |
30-60 |
Repeat infusion every 12 to 24 hours for 3-4 days or more until pain and disability have resolved. |
|
Life-threatening bleeding such as in head surgery, bleeding in the throat, major abdominal bleeding |
60-100 |
Repeat the infusion every 8 to 24 hours until the threat is resolved. |
|
Surgery | ||
|
Minor including tooth extraction |
30-60 |
Every 24 hours for at least 1 day, until healing is achieved. |
|
Major |
80-100 (before and after an operation) |
Repeat infusion every 8 to 24 hours until adequate wound healing, then therapy for at least another 7 days to maintain factor VIII activity at 30% to 60%. |
Dosage for the prevention of bleeding If you suffer from severe haemophilia A you should inject 20 to 40 III of factor VIII per kg body weight every two or three days for long-term prevention. Your dosage should be adjusted according to your response. In some cases shorter dosage intervals or higher dosages may be necessary.
Dosage calculation
Always use Octanate exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Factor VIII activity refers to the amount of factor VIII present in the plasma. It is expressed either as a percentage (relative to normal human blood plasma) or in International Units (IU). The dosage of factor VIII is expressed in IU.
One IU of factor VIII activity is eguivalent to the amount of factor VIII in one ml of normal human blood plasma. One IU of factor VIII per kg body weight raises the plasma factor VIII activity by 1.5% - 2% of normal activity. To calculate your dosage, the level of factor VIII activity in your blood plasma is measured. This will indicate by how much the activity needs to be increased. Please consult your doctor if you are uncertain how much your factor VIII activity has to be increased or how to calculate your dosage.
The dosage reguired is calculated using the following formula:
Your dosage and how often it must be administered (frequency) should always be oriented by the clinical efficacy in the individual patient.
In the following bleeding events, factor VIII activity should not fall below the plasma activity level (in % of normal) shown in the following table, for the corresponding period.
This table can be used to guide dosing in bleeding episodes and for surgery:
Your doctor will advise you about the dosage and the frequency with which you should use Octanate.
Your response to factor VIII products may vary. The factor VIII level in your blood should therefore be measured during the treatment to calculate the correct dosage and frequency of infusion. Use in children
A clinical study (including 15 patients of 6 years of age or less) did not identify any special dosage requirements for children.
Clinical data on the use of Octanate in previously untreated patients (PUPs) are limited. A clinical study is ongoing. So far 10.3% of PUPs treated with Octanate developed inhibitors. PUPs need to be tested for the possible development of antibodies (e.g. Bethesda test) when treated.
Instructions for Home Treatment
• Please read all the instructions and follow them carefully!
• Do not use Octanate after expiry date given on the label.
• During the procedure described below, sterility must be maintained!
• The solution in the syringe should be clear or slightly pearly shimmery. Do not inject solutions that are cloudy or have deposits.
• Use the prepared solution immediately, to prevent microbial contamination.
• Only use the injection set provided. The use of other injection/infusion equipment can cause additional risks and treatment failure.
Instructions for preparing the solution:
1. Do not use the product directly from the refrigerator. Allow the solvent and the powder in the closed vials to reach room temperature.
2. Remove the flip off caps from both vials and clean the rubber stoppers with one of the provided alcohol swabs.
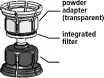
3. The Mix2vial™ is depicted in Fig. 1. Place the solvent vial on an even surface and hold it firmly. Take the Mix2Vial™ and turn it upside down. Place the blue part of the Mix2Vial™

Powder
Fig. 5

on top of the solvent vial and press firmly down until it snaps (Fig. 2+3).
solvent
adapter
(blue)


Fig. 2

injecting Octanate.
No blood must flow into the syringe due to the risk of formation of fibrin clots.
5. Inject the solution into the vein at a slow speed, not faster than 2-3 ml per minute.
4. Place the powder vial on an even surface and hold it firmly. Take the solvent vial with the attached Mix2Vial™ and turn it upside down. Place the transparent part on top of the powder vial and press firmly down until it snaps (Fig. 4). The solvent flows automatically into the powder vial.

If you use more than one vial of Octanate powder for one treatment, you may use the same injection needle and syringe again. The Mix2Vial™ is for single use only.
If you use more Octanate than you should No symptoms of overdose with human coagulation factor VIII have been reported. However, the recommend dosage should not be exceeded.
I
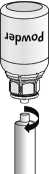
5. With both vials still attached, gently swirl the powder vial until the product is dissolved.
The dissolving is completed in less than 10 minutes at room temperature. Slight foaming might occur during preparation. Unscrew the Mix2Vial™ into two parts (Fig. 5). Foaming will disappear.
Dispose the empty solvent vial with the blue part of the Mix2Vial™.
Instructions for injection:
As a precaution, your pulse rate should be taken before and during the injection. If a marked increase in your pulse rate occurs, reduce the injection speed or interrupt the administration for a short time.
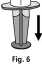
1. Attach the syringe to the transparent part of the Mix2Vial™. Turn the vial upside down and draw the solution into the syringe (Fig. 6).
The solution in the syringe should be clear or slightly pearly shimmery.
Once the solution has been transferred, firmly hold the plunger of the syringe (keeping it facing down) and remove the syringe from the Mix2Vial™ (Fig. 7). Dispose the Mix2Vial™ and the empty vial.
2. Clean the chosen injection site with one of the provided alcohol swabs.
3. Attach the provided injection needle to the syringe
4. Insert the injection needle into the chosen vein. If you have used a tourniguet to make the vein easier to see, this tourniguet should be released before you start
If you have any further guestions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
rare: affects 1 to 10 users in 10,000 very rare: affects less than 1 user in 10,000
• Even though rare, hypersensitivity or allergic reactions have been observed in patients treated with factor VIII containing products. Early signs of allergic reactions can include: being sick (vomiting), burning and stinging at the infusion site, chest tightness, chills, faster heart beat (tachycardia), feeling sick (nausea), feeling of pins and needles (tingling), flushing, headache, hives (urticaria), low blood pressure (hypotension), rash, restlessness, swelling of the face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing (angioedema), tiredness (lethargy), wheezing.
If you suffer from any of the above-mentioned symptoms, please inform your doctor.
• In very rare cases, this hypersensitivity may lead to a severe life-threatening allergic reaction called anaphylaxis, which may include shock, as well as some or all of the symptoms described above. In this case please contact your doctor immediately or call for an ambulance.
• Fever may occur in rare cases.
• If you suffer from haemophilia Ayou may develop inhibitors (neutralising antibodies) to factor VIII. If such antibodies occur, they might prevent your medicine from working properly and bleeding may continue. In such rare cases, it is recommended that a specialised haemophilia centre be contacted immediately. You should be carefully monitored for the development of inhibitors by appropriate clinical observations and laboratory tests.lnhibitors may increase the risk of suffering severe allergic reactions (anaphylactic shock). If you suffer an allergic reaction, you should be tested for the presence of an inhibitor.
• For information on viral safety see section 2. (Take special care with Octanate - Information about the blood and plasma used for Octanate).
B.120.006.UK