Octanine 500 Iu 500 Iu Powder And Solvent For Solution For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
OCTANINE 500 IU, 500 IU powder and solvent for solution for injection OCTANINE 1000 IU, 1000 IU powder and solvent for solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
• OCTANINE 500 IU is presented as a powder and solvent for solution for injection containing nominally 500 IU human coagulation factor IX per vial.
The product contains approximately 100 IU/ml human coagulation factor IX when reconstituted with 5 ml water for injections (Ph.Eur.).
• OCTANINE 1000 IU is presented as a powder and solvent for solution for injection containing nominally 1000 IU human coagulation factor IX per vial.
The product contains approximately 100 IU/ml human coagulation factor IX when reconstituted with 10 ml water for injections (Ph.Eur.).
OCTANINE is produced from plasma of human donors.
The potency (IU) is determined using the European Pharmacopoeia one stage clotting test, in comparison with an international standard from the World Health Organisation (WHO). The specific activity of OCTANINE is approximately 100 IU/mg protein.
This medicinal product contains up to 3 mmol (or 69 mg) sodium for 1 vial OCTANINE 500 IU and up to 6 mmol (or 138 mg) sodium for 1 vial OCTANINE 1000 IU per dose. To be taken into consideration by patients on a controlled sodium diet.
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Powder and solvent for solution for injection.
The powder is white or pale yellow also appearing as a friable solid.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Treatment and prophylaxis of bleeding in patients with haemophilia B (congenital factor IX deficiency).
4.2 Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of haemophilia.
Previously untreated _patients
The safety and efficacy of OCTANINE in previously untreated patients have not yet been established.
Treatment monitoring
During the course of treatment, appropriate determination of factor IX levels is advised to guide the dose to be administered and the frequency of repeated infusions. Individual patients may vary in their response to factor IX, demonstrating different half-lives and recoveries. Dose based on bodyweight may require adjustment in underweight or overweight patients. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor IX activity) is indispensable.
Posology
Dose and duration of the substitution therapy depend on the severity of the factor IX deficiency, on the location and extent of the bleeding and on the patient’s clinical condition.
The number of units of factor IX administered is expressed in International Units (IU), which are related to the current WHO standard for factor IX products. Factor IX activity in plasma is expressed either as a percentage (relative to normal human plasma) or in International Units (relative to an International Standard for factor IX in plasma).
One International Unit (IU) of factor IX activity is equivalent to that quantity of factor IX in one ml of normal human plasma.
On demand treatment
The calculation of the required dosage of factor IX is based on the empirical finding that 1 International Unit (IU) factor IX per kg body weight raises the plasma factor IX activity by 1 % of normal activity. The required dosage is determined using the following formula:
Required units = body weight (kg) x desired factor IX rise (%) (IU/dl) x 0.8
The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
In the case of the following haemorrhagic events, the factor IX activity should not fall below the given plasma activity level (in % of normal) in the corresponding period. The following table can be used to guide dosing in bleeding episodes and surgery:
|
Degree of haemorrhage / Type of surgical procedure |
Factor IX level required (%) (IU/dl) |
Frequency of doses (hours) / Duration of therapy (days) |
|
Haemorrhage | ||
|
Early haemarthrosis, muscle bleeding or oral bleeding |
20 - 40 |
Repeat every 24 hours. At least 1 day, until the bleeding episode as indicated by pain is resolved or healing is achieved. |
|
More extensive haemarthrosis, muscle bleeding or haematoma |
30 - 60 |
Repeat infusion every 24 hours for 3 - 4 days or more until pain and acute disability are resolved. |
|
Life-threatening haemorrhages |
60 - 100 |
Repeat infusion every 8 to 24 hours until threat is resolved. |
|
Surgery | ||
|
Minor Surgery including tooth extraction |
30 - 60 |
Every 24 hours, at least 1 day, until healing is achieved. |
|
Major Surgery |
80 - 100 (pre-and/post- operative) |
Repeat infusion every 8-24 hours until adequate wound healing, then therapy for at least another 7 days to maintain a factor IX activity of 30% to 60% (IU/dl). |
For long term prophylaxis against bleeding in patients with severe haemophilia B, the usual doses are 20 to 40 IU of factor IX per kilogram of body weight at intervals of 3 to 4 days.
In some cases, especially in younger patients, shorter dosage intervals or higher doses may be necessary.
Continuous infusion
There is not enough data available to recommend continous infusion of OCTANINE in surgical procedures.
Paediatric Population
In the study conducted in 25 children under 6 years of age, the median dose administered per exposure day was similar for prophylaxis and treatment of bleeding, i.e. 35 to 40 IU/kg BW.
Method of administration Intravenous use.
It is recommended not to administer more than 2 - 3 ml per minute.
For instructions on reconstitution of the medicinal product before administration, see section 6.
4.3 Contraindications
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
- Known allergy related reduction of thrombocytes during Heparin treatment (HIT type II).
4.4 Special warnings and special precautions for use
Hypersensitivity
Allergic type hypersensitivity reactions are possible with OCTANINE. The product contains traces of human proteins other than factor IX and heparin. If symptoms of hypersensitivity occur, patients should be advised to discontinue use of the medicinal product immediately and contact their physician. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalised urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis.
In case of shock, standard medical -treatment for shock should be implemented.
Inhibitors
After repeated treatment with human coagulation factor IX products, patients should be monitored for the development of neutralising antibodies (inhibitors) that should be quantified in Bethesda Units (BU) using appropriate biological testing
There have been reports in the literature showing a correlation between the occurrence of a factor IX inhibitor and allergic reactions. Therefore, patients experiencing allergic reactions should be evaluated for the presence of an inhibitor. It should be noted that patients with factor IX inhibitors may be at an increased risk of anaphylaxis with subsequent challenge with factor IX. Because of the risk of allergic reactions with factor IX products, the initial administrations of factor IX should, according to the treating physician’s judgement, be performed under medical observation where proper medical care for allergic reactions could be provided
Thromboembolism
Because of the potential risk of thrombotic complications, clinical surveillance for early signs of thrombotic and consumptive coagulopathy should be initiated with appropriate biological testing when administering this product to patients with liver disease, to patients post-operatively, to new-born infants, or to patients at risk of thrombotic phenomena or disseminated intravascular coagulation (DIC). In each of these situations, the benefit of treatment with OCTANINE should be weighed against the risk of these complications
Cardiovascular events
In patients with existing cardiovascular risk factors, substitution therapy with FIX may increase the cardiovascular risk.
Catheter-related complications
If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteraemia and catheter site thrombosis should be considered.
Transmissible agents
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens.
The measures taken are considered effective for enveloped viruses such as human immunodeficieny virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) and for the non-enveloped virus hepatitis A virus (HAV). The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g. haemolytic anaemia).
Appropriate vaccination (hepatitis A and B) should be considered for patients in regular / repeated receipt of human plasma-derived factor IX concentrates.
It is strongly recommended that every time that OCTANINE is administered to a patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
Patients on controlled sodium diet
This medicinal product contains up to 3 mmol (or 69 mg) sodium for 1 vial OCTANINE 500 IU and up to 6 mmol (or 138 mg) sodium for 1 vial OCTANINE 1000 IU per dose. To be taken into consideration by patients on a controlled sodium diet.
Paediatric population
The listed warnings and precautions apply both to adults and children.
4.5 Interaction with other medicinal products and other forms of interaction
No interactions of human coagulation factor IX products with other medicinal products have been reported.
4.6 Fertility, pregnancy and lactation
Animal reproduction studies have not been conducted with factor IX. Based on the rare occurrence of haemophilia B in women, experience regarding the use of factor IX during pregnancy and breastfeeding is not available. Therefore, factor IX should be used during pregnancy and lactation only if clearly indicated.
4.7 Effects on ability to drive and use machines
Octanine has no influence on the ability to drive and use machines.
4.8 Undesirable effects
System Organ Class
Rare
Very rare
|
Immune system disorders |
hypersensitivity reaction |
anaphylactic shock |
|
Vascular disorders |
embolism | |
|
Renal and urinary disorders |
nephrotic syndrome | |
|
General disorders and administration site conditions |
heparin induced thrombocytopenia pyrexia | |
|
Investigations |
anti factor IX antibody positive |
rare (>1/10,000 to <1/1,000)
very rare (<1/10,000), including isolated reports
• Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the infusion site, chills, flushing, generalised urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) have been observed infrequently in patients treated with factor IX containing products. In some cases, these reactions have progressed to severe anaphylaxis, and they have occurred in close temporal association with development of factor IX inhibitors (see also 4.4).
• Patients with haemophilia B may develop neutralising antibodies (inhibitors) to factor IX. If such inhibitors occur, the condition will manifest itself as an insufficient clinical response. In such cases, it is recommended that a specialised haemophilia centre be contacted. A study in 25 children with Haemophilia B was conducted, thereof 6 patients were previously untreated and had a median no. of exposure days to OCTANINE of 38 (range 8-90). All patients had a factor IX inhibitor level of <0.4 BU at baseline. No inhibitor was observed during the study.
• Nephrotic syndrome has been reported following attempted immune tolerance induction in haemophilia B patients with factor IX inhibitors and a history of allergic reaction.
• On rare occasions, fever has been observed.
• There is a potential risk of thromboembolic episodes following the administration of factor IX products, with a higher risk for low purity preparations. The use of low purity factor IX products has been associated with instances of myocardial infarction, disseminated intravascular coagulation, venous thrombosis and pulmonary embolism. The use of high purity factor IX is rarely associated with such side effects.
• Due to the amount of heparin contained, a sudden, allergy induced reduction of the blood platelet count below 100,000/pl or 50% of the starting count may be observed (thrombocytopenia type II) in rare cases. In patients not previously hypersensitive to heparin, this decrease in thrombocytes may occur 6-14 days after the start of treatment. In patients with a previous heparin hypersensitivity this reduction may set in a few hours after treatment.
This severe form of blood platelet reduction may be accompanied by, or result in, arterial and venous thrombosis, thromboembolism, severe clotting disorder (consumptive coagulopathy), skin necrosis in the area of injection, flea bitelike bleeding (petechial haemorrhages), purpura and tarry stool. If the specified allergic reactions are observed, the injections with OCTANINE should be stopped immediately. The patient should be advised not to use any heparin containing medicinal products in the future. Because of this rarely occurring heparin induced effect on the blood platelets, the patient’s blood platelet count should be monitored closely, especially at the initiation of treatment.
• For safety with respect to transmissible agents, see 4.4.
4.9 Overdose
No case of overdose has been reported.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: antihemorrhagics: blood coagulation factor IX ATC-code: B02BD04
Factor IX is a single chain glycoprotein with a molecular mass of about 68,000 Dalton. It is a vitamin-K dependent coagulation factor and it is synthesised in the liver. Factor IX is activated by factor XIa in the intrinsic coagulation pathway, and by the factor VII/tissue factor complex in the extrinsic pathway. Activated factor IX, in combination with activated factor VIII, activates factor X. Activated factor X converts prothrombin into thrombin. Thrombin then converts fibrinogen into fibrin and a clot is formed.
Haemophilia B is a sex-linked hereditary disorder of blood coagulation due to decreased levels of factor IX and results in profuse bleeding into joints, muscles or internal organs, either spontaneously or as a result of accidental or surgical trauma. By replacement therapy the plasma levels of factor IX is increased, thereby enabling a temporary correction of the factor deficiency and correction of the bleeding tendencies.
Paedriatric population
A study in 25 children below 6 years of age was conducted. Thereof, 6 patients were previously untreated. The recovery after administration of >25 IU of OCTANINE/kg body weight was investigated during the first 3 months of treatment and after 12-24 months. The incremental recovery (geometric mean ± s.d., one-stage assay, actual potency) was calculated to be
0.8±1.4 and 0.9±1.3 %/IU/kg at the 1st and the 2nd assessment, respectively.
5.2.
Pharmacokinetic properties
For OCTANINE the following results were achieved in one pharmacokinetic study with 13 Haemophilia B patients over 12 years of age (mean age 28 years, range 12-61 years):
|
N=13 |
Median |
Mean |
SD* |
Minimum |
Maximum |
|
Incremental Recovery [IU/dl]/[IU/kg] |
1.2 |
1.3 |
0.5 |
0.8 |
2.4 |
|
AUC* r-w\s norm (IU x dl-1 x h x IU-1 x kg) |
32.4 |
37.7 |
13.0 |
24.5 |
64.0 |
|
Half-life (h) |
27.8 |
29.1 |
5.2 |
22.0 |
36.8 |
|
MRT* (h) |
39.4 |
40.0 |
7.3 |
30.2 |
51.6 |
|
Clearance (ml x h-1 x kg) |
3.1 |
2.9 |
0.9 |
1.6 |
4.1 |
*AUC = area under the curve *MRT = mean residence time *SD = standard deviation
The incremental recovery was also tested in a second study. The meta-analysis of all recovery assessments (n=19) resulted in a mean recovery of 1.1 [IU/dl]/[IU/kg].
5.3. Preclinical safety data
Human plasma coagulation factor IX (from the concentrate) is a normal constituent of the human plasma and acts like the endogenous factor IX.
Animal studies are limited and show no additional risks to those already mentioned in other sections of the SmPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Powder:
Heparin,
Sodium chloride,
Sodium citrate,
Arginine hydrochloride,
Lysine hydrochloride
Solvent:
water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
Only the provided injection/infusion sets should be used because treatment failure can occur as a consequence of human coagulation factor IX adsorption to the internal surfaces of some injection/infusion equipment.
6.3 Shelf life
2 years
Chemical and physical in use stability has been demonstrated for 72 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution / dilution has taken place in controlled and validated aseptic conditions.
6.4 Special precautions for storage
Do not store above 25°C.
Do not freeze.
Keep the vial in the outer carton in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5 Nature and contents of container
OCTANINE comes as a combination package consisting of two cartons held together with a plastic film.
OCTANINE 500 IU:
Carton 1: powder in a 30 ml vial (type I glass), with a stopper (chlorobutyl or bromobutyl rubber) and a flip off cap (aluminium); package leaflet.
+
Carton 2: 5 ml of solvent (water for injections) (type I or type II glass), with a stopper (chlorobutyl or bromobutyl rubber) and a flip off cap (aluminium).
OCTANINE 1000 IU:
Carton 1: powder in a 30 ml vial (type I glass), with a stopper (chlorobutyl or bromobutyl rubber) and a flip off cap (aluminium); package leaflet.
+
Carton 2: 10 ml of solvent (water for injections) (type I or type II glass), with a stopper (chlorobutyl or bromobutyl rubber) and a flip off cap (aluminium).
Carton 2 also contains the following medical devices:
• 1 disposable syringe
• 1 transfer set Mix2Vial™
• 1 infusion set (butterfly)
• 2 alcohol swabs
6.6 Special precautions for disposal and other handling
Please read all the instructions and follow them carefully!
Do not use Octanine after expiry date given on the label and carton.
During the procedure described below, sterility must be maintained!
The solution in the syringe should be clear or slightly pearly shimmery. Do not inject solutions that are cloudy or have deposits.
Use the prepared solution immediately, to prevent microbial contamination.
Only use the injection set provided. The use of other injection/infusion equipment can cause additional risks and treatment failure.
Instructions for preparing the solution:
1. Do not use the product directly from the refrigerator. Allow the solvent and the powder in the closed vials to reach room temperature.
2. Remove the flip off caps from both vials and clean the rubber stoppers with one of the provided alcohol swabs.
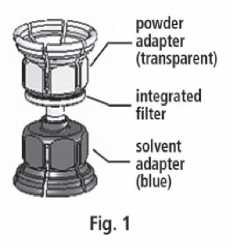
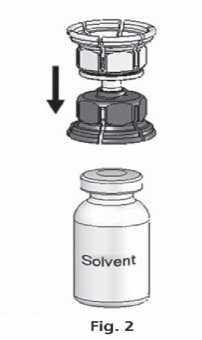
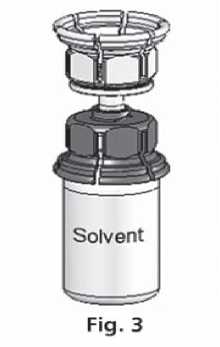
3. The Mix2vial™ is depicted in Fig. 1. Place the solvent vial on an even surface and hold it firmly. Take the Mix2Vial™ and turn it upside down. Place the blue part of the Mix2Vial™ on top of the solvent vial and press firmly down until it snaps (Fig. 2+3).

4. Place the powder vial on an even surface and hold it firmly. Take the solvent vial with the attached Mix2Vial™ and turn it upside down. Place the transparent part on top of the powder vial and press firmly down until it snaps (Fig. 4). The solvent flows automatically into the powder vial.

5. With both vials still attached, gently swirl the powder vial until the product is dissolved.
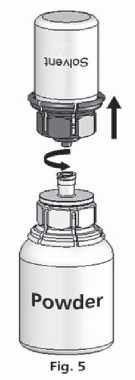
The dissolving is completed in less than 10 minutes at room temperature. Slight foaming might occur during preparation. Unscrew the Mix2Vial™ into two parts (Fig. 5). Foaming will disappear.
Dispose the empty solvent vial with the blue part of the Mix2Vial™.
As a precaution, your pulse rate should be taken before and during the injection. If a marked increase in your pulse rate occurs, reduce the injection speed or interrupt the administration for a short time.
1. Attach the syringe to the transparent part of the Mix2Vial™. Turn the vial upside down and draw the solution into the syringe (Fig. 6).
The solution in the syringe should be clear or slightly pearly shimmery.
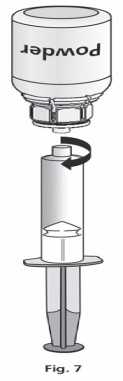
Once the solution has been transferred, firmly hold the plunger of the syringe (keeping it facing down) and remove the syringe from the Mix2Vial™ (Fig. 7). Dispose the Mix2Vial™ and the empty vial.
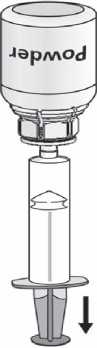
Fig. 6
2. Clean the chosen injection site with one of the provided alcohol swabs.
3. Attach the provided injection needle to the syringe
4. Insert the injection needle into the chosen vein. If you have used a tourniquet to make the vein easier to see, this tourniquet should be released before you start injecting Octanine.
No blood must flow into the syringe due to the risk of formation of fibrin clots.
5. Inject the solution into the vein at a slow speed, not faster than 2-3 ml per minute.
If you use more than one vial of Octanine powder for one treatment, you may use the same injection needle and syringe again. The Mix2Vial™ is for single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Octapharma Ltd The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 10673/0015
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
16/03/2010
10 DATE OF REVISION OF THE TEXT
24/02/2016