Octaplex 1000 Iu Powder And Solvent For Solution For Injection
|
Initial INR |
2-2.5 |
2.5-3 |
3-3.5 |
> 3.5 |
|
Approximate dose* (ml Octaplex/kg body weight) |
0.9-1.3 |
1.3-1.6 |
1.6-1.9 |
>1.9 |
*The single dose should not exceed 3,000 lU (= 120 ml Octaplex).
Dose
Bleeding and prevention of bleeding during vitamin K antagonist treatment:
The dose will depend on the Internal normalised ratio (INR) before treatment and the targeted INR. In the following table approximate doses (ml/kg body weight of the reconstituted product) reguired for normalisation of INR (< 1.2 within 1 hour) at different initial INR levels are given.
As these recommendations are empirical and recovery and the duration of effect may vary, monitoring of INR during treatment is mandatory.
Bleeding and perioperative prophylaxis in congenital deficiency of the vitamin K dependent coagulation factors II and X when specific coagulation factor product is not available:
The calculated reguired dosage for treatment is based on the empirical finding that approximately 1 IU of factor II or X per kg body weight raises the plasma factor II or X activity by 0.02 and 0.017 lU/ml, respectively.
• Reguired units = body weight (kg) x desired factor X rise (lU/ml) x 59 where 59 (ml/kg) is the reciprocal of the estimated recovery.
• Reguired dosage for factor II:
Reguired units = body weight (kg) x desired factor II rise (lU/ml) x 50 If the individual recovery is known that value should be used for calculation.
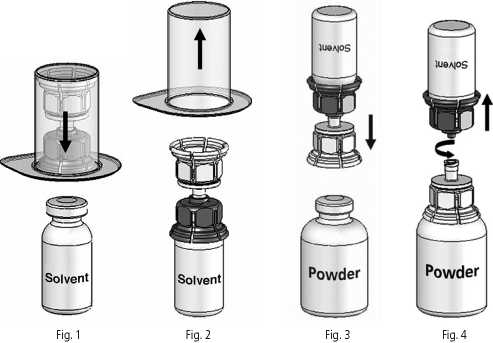
Instructions for reconstitution:
1. If necessary, allow the solvent (Water for Injections) and the powder in the closed vials to reach room temperature.This temperature should be maintained during reconstitution. If a water bath is used for warming, care must be taken to avoid water coming into contact with the rubber stoppers or the caps of the vials. The temperature of the water bath should not exceed 37°C.
2. Remove the caps from the powder vial and the water vial and clean the rubber stoppers with an alcohol swab.
3. Peel away the lid of the outer package of the Mix2Vial™. Place the solvent vial on an even surface and hold it firmly. Place the blue part of the Mix2Vial™ on top of the solvent vial and press firmly down until it snaps (Fig. 1). While holding onto the solvent vial, carefully remove the outer package from the Mix2Vial™, being careful to leave the Mix2Vial™ attached firmly to the solvent vial (Fig. 2).
4. Place the powder vial on an even surface and hold it firmly. Take the solvent vial with the attached Mix2Vial™ and turn it upside down. Place the transparent part on top of the powder vial and press firmly down until it snaps (Fig. 3).The solvent flows automatically into the powder vial.
5. With both vials still attached, gently swirl the powder vial until the product is dissolved. Octaplex dissolves guickly at room temperature to a colourless to slightly blue solution. Unscrew the Mix2Vial™ into two parts (Fig. 4).
Dispose the empty solvent vial with the blue part of the Mix2Vial™.
If the powder fails to dissolve completely or an aggregate is formed, do not use the preparation.
Instructions for infusion:
As a precautionary measure, the patients pulse rate should be measured before and during the infusion. If a marked increase in the pulse rate occurs the infusion speed must be reduced or the administration must be interrupted.
1. Attach a 40 ml syringe to the transparent part of the Mix2Vial™.Turn the vial upside down and draw the solution into the syringe.
Once the solution has been transferred, firmly hold the plunger of the syringe (keeping it facing down) and remove the syringe from the Mix2Vial™.
Dispose the Mix2Vial™ and the empty vial.
2. Disinfect the intended injection site with an alcohol swab.
3. Inject the solution intravenously at a slow speed: Initially 1 ml per minute, not faster than 2 - 3 ml per minute.
No blood must flow into the syringe due to the risk of formation of fibrin clots.The Mix2Vial™ is for single use only.
B.261.000.UK

Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Octaplex 1000 III
powder and solvent for solution for infusion Human prothrombin complex
What is in this leaflet
1. What Octaplex is and what it is used for
2. What you need to know before you use Octaplex
3. How to use Octaplex
4. Possible side effects
5. How to store Octaplex
6. Contents of the pack and other information
1. WHAT OCTAPLEX IS AND WHAT IT IS USED FOR
Octaplex belongs to a group of medicines called clotting factors. It contains the human vitamin K dependent blood coagulation factors II, VII, IX and X.
Octaplex is used to treat and prevent bleeding:
• caused by medicines called vitamin K antagonists (such as warfarin). These medicines block the effect of vitamin K and cause a shortage of the vitamin K dependent clotting factors in your body. Octaplex is used when rapid correction of the shortage is required.
• in people born with a shortage of the vitamin K dependent clotting factors II and X. It is used when purified specific clotting factor product is not available.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE OCTAPLEX
Octaplex must not be used:
• if you are allergic to one of the ingredients of this product (listed in section 6).
• if you are allergic to heparin or if heparin has ever caused a reduction in the level of platelets in your blood.
Warnings and precautions
• Take the advice of a doctor who specialises in clotting disorders, when receiving Octaplex.
• If you have an acquired deficiency of the vitamin K dependent clotting factors (for example B,261.000.UK
caused by treatment with vitamin K antagonist medicines), Octaplex should only be used when rapid correction of the shortage is necessary such as major bleeding or emergency surgery. In other cases, lowering the dose of the vitamin K antagonist medicine and/or administration of vitamin K is usually sufficient.
If you receive a vitamin K antagonist medicine (like warfarin) you may have an increased risk of forming blood clots. In this case, treatment with Octaplex may enhance the risk.
If you have been born with a shortage of any of the vitamin K dependent factors, specific coagulation factor product should be used when available.
If an allergic or anaphylactic- type reaction occurs, your doctor will stop the infusion immediately and give appropriate treatment.
There is a risk of thrombosis or disseminated intravascular coagulation (serious illness, with clots forming all over the body) when you receive Octaplex (particularly if you receive it regularly).You should be observed closely for signs or symptoms of intravascular coagulation or thrombosis.
This is especially important if you have a history of coronary heart disease, liver disease, if you are going to have an operation and also if Octaplex is given to very small babies No data are available regarding the use of Octaplex in case of bleeding during the birth due to vitamin K deficiency in the new born.
Viral Safety
• When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients.These include careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded, and the testing of each donation and pools of plasma for signs of virus/infections. Manufacturers of these products also include steps in the processing of the blood or plasma that can inactivate or remove viruses. Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded.This also applies to any unknown or emerging virus or other types of infections. The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus.The measures taken may be of limited value against non-enveloped viruses such as hepatitis A virus and parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (foetal infection) and for individuals whose immune system is depressed or who suffer from a type of anaemia (e.g. sickle cell disease or haemolytic anaemia).
It is strongly recommended that every time you receive a dose of Octaplex, the name and batch number of the product are recorded in order to maintain a link to the batches used.
• Appropriate vaccination (hepatitis A and B) is recommended for you if you receive human plasma-derived prothrombin complex products regularly/repeatedly.
Other medicines and Octaplex
Octaplex must not be mixed with other medicinal products.
Octaplex stops the effect of vitamin K antagonist medicines (like Warfarin), but no interactions with other medicines are known.
Octaplex may affect the results of clotting tests which are sensitive to heparin.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding
Octaplex should only be used during pregnancy and breast-feeding if clearly needed. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
It is not known how Octaplex affects the ability to drive and use machines.
Important information about some of the ingredients of Octaplex
• Heparin may cause allergic reactions and reduced blood cell counts which may affect the blood clotting system. Patients with a history of allergic reactions caused by heparin should not use heparin-containing medicines.
• Octaplex contains 150 - 250 mg sodium per vial.To be taken into consideration by patients on a controlled sodium diet.
3. HOW TO USE OCTAPLEX
Treatment with Octaplex should be started under the supervision of a doctor who is specialised in clotting disorders.
• First, the powder is dissolved in water
• Then the solution is given into a vein (the intravenous route).
How much Octaplex you receive, and for how long, depends on:
• how serious your illness is;
• where the bleeding is and how severe it is, and
• your general condition.
If you got more Octaplex than you should
In case of overdose, the risk is higher of developing
• clotting complications (such as heart attack and clots in your veins or lungs)
• disseminated intravascular coagulation (a serious illness where clots form all over the body).
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
Some patients may have allergic type reactions and fever.
Immune system problems:
Rare (more than 1 of 10,000, but less than 1 of 1,000 patients)
Rarely, patients treated with Octaplex for replacement therapy may develop neutralising antibodies (inhibitors) against any of the contained clotting factors. If such inhibitors occur, the replacement therapy will not be very effective.
General problems
Rare (more than 1 of 10,000, but less than 1 of 1,000 patients)
Increase in body temperature (fever) has not been observed but may rarely occur.
Vascular disorders
There is a risk of blood clotting following the administration of this medicine.
Nervous system disorders
Rare (more than 1 of 10,000, but less than 1 of 1,000 patients)
Headache may rarely occur.
Investigations
Rare (more than 1 of 10,000, but less than 1 of 1,000 patients)
A temporary increase in liver test results (transaminases) has been rarely observed.
Others
The heparin in the preparation may cause a sudden fall in the number of platelets in the blood. This is an allergic reaction called "heparin-induced thrombocytopenia type II". In rare cases in patients not previously hypersensitive to heparin, this fall in the number of platelets can occur 6-14 days after the start of treatment. In patients with a previous heparin hypersensitivity, this alteration may develop within a few hours of starting treatment.
The treatment with Octaplex must be stopped immediately in patients showing this allergic reaction. These patients must not receive heparin containing medicinal products in the future.
For information on viral safety see section 2.
Reporting of suspected adverse reactions
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE OCTAPLEX
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last day of that month.
Do not store above 25 °C. Do not freeze. Store in the original package in order to protect from light.
B.261.000.UK
The powder should be dissolved only directly before injection.The stability of the solution has been demonstrated for up to 8 hours at +25°C. Nevertheless, to prevent contamination, the solution should be used immediately and on one occasion only.

6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Octaplex contains, per vial and after reconstitution with 40 ml solvent
The active substances are:
|
Name of ingredient |
Octaplex Quantity per vial |
Octaplex Quantity per ml reconstituted solution |
|
Total protein: |
520-1640 mg |
13-41 mg/ml |
|
Active substances | ||
|
Human coagulation factor II |
560- 1520 IU |
14-38 lU/ml |
|
Human coagulation factorVII |
360-960 IU |
9-24 lU/ml |
|
Human coagulation factor IX |
1000IU |
25 lU/ml |
|
Human coagulation factor X |
720- 1200 IU |
18-30 lU/ml |
|
Further active ingredients | ||
|
Protein C |
520- 1240 IU |
13-31 lU/ml |
|
Protein S |
480-1280 IU |
12-32 lU/ml |
The specific activity of the product is > 0.6 lU/mg proteins, expressed as factor IX activity.
The other ingredients are:
Heparin, tri-sodium citrate dihydrate, Water for Injections.
What Octaplex looks like and contents of the pack
Octaplex is presented as a powder and solvent for solution for infusion.
Octaplex is sold in one carton containing
• 1 vial with powder for solution for infusion
• 1 vial with the solvent, 40 ml Water for Injections
• 1 transfer set Mix2 Via I
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Octapharma Limited
The Zenith Building, 26 Spring Gardens, Manchester M2 1AB, United Kingdom
For any further information about this medicinal product, please contact the local representative of the Marketing Authorisation Holder:
Octapharma Limited
The Zenith Building, 26 Spring Gardens, Manchester M2 1 AB, United Kingdom
Manufacturers:
Octapharma Pharmazeutika Produktionsges.m.b.H. Oberlaaer Str. 235,1 100 Vienna, Austria
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Belgium, Bulgaria, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, The Netherlands, Norway, Poland, Portugal, Republic of Slovenia, Slovak Republic, Spain, United Kingdom: Octaplex Czech Republic, Sweden: Ocplex Italy, Romania: Pronativ
This leaflet was last revised in 07/2015.
INFORMATION FOR HEALTHCARE PROFESSIONALS
General information about how to use Octaplex is provided in section 3.
The following information is intended for medical or healthcare professionals only:
Instructions for Treatment
Please read all the instructions and follow them carefully.
During the procedure described below, aseptic technique must be maintained.
The product reconstitutes quickly at room temperature.
The reconstituted solution should be clear or slightly opalescent.
Do not use solutions that are cloudy or have deposits.
Reconstituted products should be inspected visually for particulate matter and discoloration prior to administration.
After reconstitution the solution must be used immediately.
Any unused product or waste material should be disposed of in accordance with local requirements.
5 6