Oftaquix Unit Dose 5Mg/Ml Eye Drops
20193/3
PACKAGE LEAFLET: INFORMATION FOR THE USER OFTAQUIX® UNIT DOSE 5 mg/ml EYE DROPS Levofloxacin
Read all of this leaflet carefully before you start using this
medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Oftaquix® is and what it is used for
2. Before you use Oftaquix®
3. How to use Oftaquix®
4. Possible side effects
5. How to store Oftaquix®
6. Further information
1. WHAT OFTAQUIX® IS AND WHAT IT IS USED FOR
Levofloxacin is an antibiotic of the type called fluoroquinolones (sometimes shortened to quinolones). It works by killing some types of bacteria that can cause infections.
When levofloxacin is given in eye drops, it is used in children aged 1 year or over and in adults to treat bacterial infections that affect the front surfaces of the eye. Oftaquix is not recommended to be used in children below age 1 year. One type of infection in this area is called conjunctivitis, which is an infection of the covering of the front of the eye.
2. BEFORE YOU USE OFTAQUIX®
Do not use Oftaquix®
- if you are allergic (hypersensitive) to levofloxacin or other quinolones or any of the other ingredients of Oftaquix®
If you are not sure, ask your doctor or pharmacist first.
Take special care with Oftaquix®
- if an allergic reaction occurs even after a single dose stop using the medicine
- if you observe worsening of your eye symptoms during treatment, please contact your doctor as soon as possible
- if you do not see any sign of recovery within a certain treatment period agreed with your doctor, please contact your doctor as soon as possible
- generally, no types of contact lenses should be worn when the eye is infected.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
In particular, tell your doctor or pharmacist if you are applying any other type of eye drop or eye ointment before you start to use Oftaquix®.
If you are using other eye drops, you should wait at least 15 minutes between applying Oftaquix® and any other type of eye drop.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Oftaquix® eye drops should be used during pregnancy only if the potential benefit justifies the potential risk to the growing baby. Although very small amounts of levofloxacin reach the blood and the breast milk, respectively, after instilling eye drops it is very unlikely that the eye drops would harm the growing baby.
Your doctor is informed about the potential risk and will advice you whether to take Oftaquix® eye drops in this case.
Driving and using machines
Oftaquix® has minor influence on the ability to drive and use machines.
If the eye drops cause blurring of your sight when you use them, you should wait until this clears before driving or operating machinery.
3. HOW TO USE OFTAQUIX®
Always use Oftaquix® exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Oftaquix® eye drops are for ocular use and have to be applied to the outer surface of the eye.
For patients above 1 year old, the usual dose is as following:
DAYS 1 - 2
• Use one to two drops in the affected eye(s) every two hours.
• Use a maximum of 8 times per day.
DAYS 3 - 5
• Use one to two drops in the affected eye(s).
• Use a maximum of 4 times per day.
In elderly patients no adjustment of the usual dose is required.
The usual total treatment course is five days. Your doctor will advise you how long to apply the drops.
If you are putting any other medicine in your eye, you should wait at least 15 minutes between applying the different types of drops.
Use in children
No dosage modification is required in children > 1 year old.
Oftaquix is not recommended to be used in children aged below 1 year.
Before applying the drops:
If possible, ask someone else to apply the drops for you. Ask them to read these instructions with you before applying the drops.
1) Wash your hands.
2) Open the pouch along the dashed line.
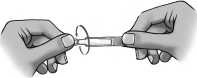
3) Remove one single-dose container from the strip.
4) Put the remaining strip back in the pouch and fold the edge to close the pouch.
5) Make sure that the solution is in the bottom
part of the single dose-container.
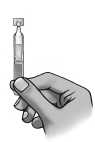
6) To open the container, twist off the tab.
7) Tilt your head backwards while seated, or lie down on your back.
8) Place the tip of the container close to your eye.
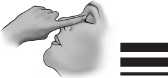
9) Pull the lower eyelid downwards and look up.
10) Press the container slightly and let one drop fall into the space between the lower eyelid and the eye.

11) Close your eye for a moment. Press the inner corner of the eye with your index finger for about one minute. Thus you can prevent the eye drop from draining down the tear duct.
If another drop is needed, and when both eyes are to be treated, m
repeat steps 8 to 11.
Oftaquix® eye drops should not be injected into the interior part of the eyeball.
For single use only. The eye drops should be used immediately after first opening the single dose container.
The contents of one single-dose container are sufficient for both eyes.
Discard container with any remaining solution after single use.
If you use more Oftaquix® than you should
If you use more Oftaquix® than you should, flush the eye(s) with water and tell your doctor or pharmacist.

If you forget to use Oftaquix®
If you forget to use the eye drops, put in the next dose as soon as you remember. Do not use a double dose to make up for a forgotten dose.
If you swallow Oftaquix® by accident
The amount of levofloxacin in the provided amount of single-dose containers is too small to cause side effects. However, if you are concerned, tell your doctor or pharmacist, who will advise you on any necessary measures.
If you stop using Oftaquix earlier than instructed, it may delay the healing process.
If you have any further questions on the use of this product, ask your doctor or the other healthcare professionals.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Oftaquix® can cause side effects, although not everybody gets them.
About one in ten people have a side effect when using Oftaquix®. Most of these affect only the eye and may not last very long. If you have any severe or persistent side effect you should stop using this eye drops and seek urgent advice from your doctor.
The frequency of possible side effects is defined as following:
|
very common: |
affects more than 1 user in 10 |
|
common: |
affects 1 to 10 users in 100 |
|
uncommon: |
affects 1 to 10 users in 1,000 |
|
rare: |
affects 1 to 10 users in 10,000 |
|
very rare: |
affects less than 1 user in 10,000 |
|
not known: |
frequency cannot be estimated from the available data |
It is possible, even though rare, to develop an allergic reaction to Oftaquix® even after just one dose. You notice this because your eyes may become red or itchy, or the lids may be swollen. If this should happen, stop using Oftaquix and contact your doctor immediately.
Common side effects (affect 1 to 10 users in 100)
• burning feeling in the eye
• decreased vision or mucus in the eye
Uncommon side effects (affect 1 to 10 users in 1,000)
• stinging or irritation of the eyes
• painful eyes
• dry or sore eyes
• swelling or redness (bloodshot eyes) of the conjunctivae (front covering of the eye)
• sensitivity to light
• itchy eyes
• sticky eyelids
• headache
• rash around the eye
• stuffed or runny nose
Rare side effects (affect 1 to 10 users in 10,000)
• allergic reactions such as skin rash
Very rare side effects (affect less than 1 user in 10,000)
• swelling and tightness in the throat
• breathing difficulty
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE OFTAQUIX®
Keep out of the reach and sight of children.
Do not use Oftaquix® after the expiry date which is stated on the single dose container, pouch and carton after “EXP”. The expiry date refers to the last day of that month.
Oftaquix® containers must be stored in the original pouch in order to protect from light.
You can use Oftaquix® 3 months after first opening the pouch. Discard any unused single dose container after that time.
Discard the opened single dose container with any remaining solution after first use immediately.
Do not use Oftaquix if you notice insoluble parts in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. FURTHER INFORMATION
What Oftaquix® contains
- The active substance is levofloxacin.
1 ml contains 5.12 mg of levofloxacin hemihydrate equivalent to 5 mg of levofloxacin.
One single-dose container (0.3 ml) contains 1.5 mg of levofloxacin.
- The other ingredients are sodium chloride, diluted sodium hydroxide solution or diluted hydrochloric acid and water for injections.
What Oftaquix® looks like and contents of the pack
- Oftaquix® is a clear, light yellow to light greenish-yellow solution, free of visible particulate matter.
- Single-dose containers of strip of ten are packed in paper-coated, aluminium-polyethylene foil pouch.
- One single-dose container contains 0.3 ml of solution.
- Pack sizes: 10 x 0.3 ml, 20 x 0.3 ml, 30 x 0.3 ml and 60 x 0.3 ml. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Santen Oy Niittyhaankatu 20 33720 Tampere Finland
For any information about this medicinal product, please contact
the local representative of the Marketing Authorisation Holder:
Kestrel Ophthalmics Ltd
7 Moor Road
Broadstone
Dorset
BH18 8AZ
United Kingdom
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Denmark |
Oftaquix 5 mg/ml ojendraber, oplosning i enkeltdosisbeholder |
|
Finland |
Oftaquix 5 mg/ml silmatipat, liuos kerta-annospipetissa |
|
Germany |
Oftaquix sine 5 mg/ml Augentropfen im Einzeldosisbehaltnis |
|
Iceland |
Oftaquix 5 mg/ml Augndropar, lausn stakskammtaila |
|
Italy |
Oftaquix 5 mg/ml collirio, soluzione, contenitore monodose |
|
Sweden |
Oftaquix 5 mg/ml ogondroppar, losning i endosbehallare |
|
United Kingdom |
Oftaquix Unit Dose 5 mg/ml Eye Drops |
This leaflet was last approved in 12/2014
§anten


282073 20193-3 Oftaquix.indd 2 11.2.2015 8.37