Oliclinomel N8-800 Emulsion For Infusion
Package leaflet: information for the user
OLICLINOMEL N8-800, Emulsion for Infusion
Read all of this leaflet carefully before you are given this medicine, because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or nurse.
• If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What OLICLINOMEL N8-800, emulsion for infusion is and what it is given for
2. What you need to know before you are given OLICLINOMEL N8-800, emulsion for infusion
3. How you will be given OLICLINOMEL N8-800, emulsion for infusion
4. Possible side effects
5. How to store OLICLINOMEL N8-800, emulsion for infusion
6. Contents of pack and other information
1. What OLICLINOMEL N8-800, emulsion for infusion is and what it is given for
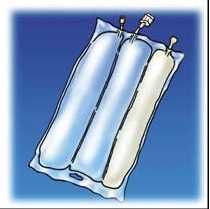
OLICLINOMEL N8-800 is an emulsion for infusion. It is presented in a bag with 3 chambers.
One chamber contains a glucose solution, the second one contains a lipid emulsion and the third one contains an amino acid solution.
Pharmacotherapeutic group: Solutions for parenteral nutrition/combinations
OLICLINOMEL N8-800 is given to provide nutrition in adults and children greater than two years of age by a tube into a vein when normal feeding by mouth is not suitable.
OLICLINOMEL N8-800 must only be given under medical supervision.
2. What you need to know before you are given OLICLINOMEL N8-800, emulsion for infusion Do not use OLICLINOMEL N8-800 and tell your doctor if:
• the patient is a premature baby, an infant or a child less than 2 years old.
• you are hypersensitive (allergic) to egg, soybean or peanuts proteins or to any other ingredient of OLICLINOMEL N8-800.
• your body has problems using amino acids.
• you have especially high level of fats in your blood (hyperlipidaemia).
• you have severe hyperglycaemia (too much sugar in your blood).
In all cases, your doctor will base his/her decision on whether you should receive this medicine on factors such as age, weight and clinical condition, together with the results of any tests performed.
Warnings and precautions
Talk to your doctor or nurse before OLICLINOMEL N8-800 is given to you.
If you are given total parenteral nutrition (TPN) solutions too fast this may result in death.
If any abnormal signs or symptoms of an allergic reaction develop, such as fever, chills, skin rashes or difficulty in breathing, excessive sweating, nauseas and headache, the infusion will be stopped immediately. This medicinal product contains soybean oil and egg phosphatide proteins. Soybean and egg proteins may cause hypersensitivity reactions. Cross-allergic reactions between soybean and peanut proteins have been observed.
Difficulty breathing could also be a sign that small particles have formed, blocking blood vessels in the lungs (pulmonary vascular precipitates). If you experience any difficulty breathing, tell your doctor or nurse. They will decide a course of action to be taken.
Y our physician will be checking and monitoring the levels of your triglycerides (a type of fat found in blood).
Certain medications and illnesses can increase the risk of developing infection or sepsis (bacteria in the blood). There is a particular risk of infection or sepsis when a tube (intravenous catheter) is placed in your vein. Your doctor will carefully watch you for any signs of infection. Patients who require parenteral nutrition (giving nutrition through a tube in your vein) can be more likely to develop infection from their medical conditions. Using “aseptic technique” (“germ free”) techniques when placing and maintaining the catheter and when making the nutritional formula can reduce the risk of infection.
Your doctor should be aware of:
• a severe kidney problem. Y ou also must inform your doctor if you are on dialysis (artificial kidney) or if you have another form of blood cleaning treatment
• a severe liver problem
• a blood clotting problems
• adrenal glands that are not working properly (adrenal insufficiency). The adrenal glands are triangle-shaped glands located on top of your kidneys
• heart failure
• lung disease
• a build up of water in your body (hyperhydration)
• not enough water in your body (dehydration)
• high blood sugar (diabetes mellitus) that you are not being treated for
• a heart attack or shock due to a sudden heart failure
• a severe metabolic acidosis (when the blood is too acid)
• a generalised infection (septicaemia)
• coma
If the patient is a child, the doctor will closely check their fluid status and/or blood values.
Fat overload syndrome has been reported with similar products. The reduced or limited ability of the body to remove the fats contained in OLICLINOMEL N8-800 may result in a "fat overload syndrome" (see section 4 - Possible Side Effects).
No additions should be made to the bag without first checking the compatibility. Formation of particles or a breaking down of the lipid emulsion could result. This can lead to blockage of the blood vessels.
If your blood sugar gets too high, your doctor should adjust the rate of OLICLINOMEL N8-800 delivery or give you insulin.
If you are severely malnourished such that you need to receive feedings by vein, it is recommended that parenteral nutrition is started slowly and carefully.
The balance of water and salt in your body and metabolic disorders will be corrected before starting the infusion. Your doctor will monitor your condition while you receive this medicine and may change the dosage or give you additional nutrients such as vitamins, electrolytes and trace elements if he/she feels they are appropriate.
To check the effectiveness and ongoing safety of the administration, your doctor will perform clinical and laboratory tests while you are receiving this medicine. If you are given this medicine for several weeks, your blood will be monitored on a regular basis. These tests are particularly required in case you suffer from certain conditions, such as a liver disorder, a kidney disorder, a disorder whereby amino acids cannot be processed by the body, a disorder in which the blood becomes too acidic, a disorder in which the level of fats and cholesterol is higher than normal, diabetes, or if you suffer from anaemia or difficulty to stop bleeding.
During the infusion if you notice pain, burning, stiffness, swelling or skin discoloration at the infusion site, or leakage of the infusion, tell your doctor or nurse. The administration will be stopped immediately and restarted in another vein.
Children
If the patient is a child, special care will be taken to give the correct dosage. Vitamin and trace element supplementation may be required depending on dose and duration. Increased precautions will also be taken because of the greater sensitivity of children to the risk of infection.
Other medicines and OLICLINOMEL N8-800
Tell your doctor or nurse if you are taking, have recently taken or might take any other medicines.
OLICLINOMEL N8-800 must not be administered together with blood through the same infusion tubing.
The olive and soybean oils present in OLICLINOMEL N8-800 contain vitamin K. This does not normally affect blood thinning medicines (anticoagulants) like coumarin. However, if you take anticoagulant medicines you should tell your doctor.
The lipids contained in this emulsion may interfere with the results of certain laboratory tests if the blood sample is taken before the lipids have been eliminated (these are generally eliminated after a period of 5 to 6 hours without receiving lipids).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before this medicine is given to you.
3. How you will be given OLICLINOMEL N8-800, emulsion for infusion
OLICLINOMEL N8-800 should only be given in adults and children greater than 2 years of age.
It is an emulsion for infusion that is given through a plastic tube into a large vein in your chest only. The prescription may be continued for as long as it is needed, depending upon your clinical condition.
OLICLINOMEL N8-800 is for single use only.
Dosage - Adults
Your doctor will decide the amount you are given depending on your individual needs and clinical condition.
The maximum daily dose is 40 ml of emulsion/kg of body weight. For example: if you weigh 70 kg, the maximum daily dose should not exceed 2800 ml of emulsion (40 ml of emulsion times 70 kg).
Dosage - Children greater than two years of age
Your doctor will decide the dose the child will need and for how long it will be given. This will depend on age, weight, height, clinical condition, daily fluid volume, energy and nitrogen requirements.
The maximum daily dose is 60 ml of emulsion/kg of body weight. For example: if the patient is a child weighing 30 kg, the maximum daily dose should not exceed 1800 ml of emulsion (60 ml of emulsion times 30 kg).
If you are given more OLICLINOMEL N8-800, emulsion for infusion than you should
If the dose given is too high or the infusion too fast, the amino acid content may make your blood too acid and you may have too much fluid in the circulation. The glucose content may increase the glucose in your blood and urine or the lipid content may increase the triglycerides in your blood. Giving a volume of OLICLINOMEL N8-800 that is too large may cause nausea, vomiting, chills and electrolyte disturbances, in such situations the infusion should be stopped immediately.
In some severe cases, your doctor may have to give you temporary renal dialysis to help your kidneys eliminate the excess product.
To prevent these events occurring, your doctor will regularly monitor your condition and test your blood parameters.
If you have any further questions on the use of this product, ask your doctor.
4. Possible side effects
Like all medicines, OLICLINOMEL N8-800 can cause side effects, although not everybody gets them. If you notice any changes in the way you feel during or after the treatment, tell your doctor or nurse right away.
The tests your doctor will perform while you are taking the medicine are meant to minimise side effects.
If any abnormal signs or symptoms of an allergic reaction develop, such as raised body temperature, chills, skin rashes or breathing difficulties, excessive sweating, nauseas and headache, the infusion should be stopped immediately.
The following side effects have been reported with OLICLINOMEL:
Common (may affect up to 1 in 10 people):
• Allergic reactions
• Headache
• Diarrhoea
• Infusion site pain, swelling
• Accumulation of fluid at infusion site
• Abnormal blood test for the liver function
• Increased blood triglycerides (fat)
Frequency not known (cannot be estimated from the available data):
• Constriction of the airway, breathing with whistling sound and/or cough (bronchospasm as part of allergic reaction)
• Fever
• Tremor
• Abdominal pain, chest pain, back pain, extremity pain
• Vomiting, nausea
• Increase in the size of the liver (hepatomegaly)
• Jaundice (yellowish of the skin or white of the eyes caused by liver or blood problem)
• Abnormal flushing of the skin (erythema)
• Excessive sweating
• Decrease in the number of platelets (reduction in number of cells in charge of blood clotting which causes bleeding as nosebleed)
• Increased blood glucose (sugar)
• Death of tissue cells (necrosis) around the infusion site
• A local defect of tissue, which can lead to necrosis (ulcer)
The following side effects have been reported with similar products:
Very rare (may affect up to 1 in 10000 people):
The reduced or limited ability to remove the lipids contained in OLICLINOMEL N8-800 may result in a "fat overload syndrome". This may be caused by overdose but may also occur at the start of an infusion, even if the product is administered according to instructions. It is associated with a sudden deterioration in the patient's clinical condition. It is characterised by high level of fats in your blood, fever, liver fatty infiltration (high level of fat in your liver), and/or an increase of your liver volume, an anaemia (reduction in red blood cells which can make the skin pale and cause weakness or breathlessness), a fall in white blood cells and blood platelets, problem with your blood clotting and/or coma may also occur. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped.
Frequency not known (cannot be estimated from the available data)
Formation of small particles which may lead to blockage of blood vessels in the lungs (pulmonary vascular precipitates) resulting in pulmonary vascular embolism and difficulty breathing (respiratory distress).
Fall in white blood cells and blood platelets have been reported in children.
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store OLICLINOMEL N8-800, emulsion for infusion
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the container and the outer packaging after EXP. This expiry date refers to the last day of that month.
Do not freeze.
Keep the container in the outer carton in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What OLICLINOMEL N8-800, emulsion for infusion contains
The active substances for each bag of the reconstituted emulsion are:
|
Active substances |
2000 ml |
|
Refined soya bean oil + refined olive oil * |
60.00 g |
|
L-alanine |
20.70 g |
|
L-arginine |
11.50 g |
|
Glycine |
10.30 g |
|
L-histidine |
4.80 g |
|
L-isoleucine |
6.00 g |
|
L-leucine |
7.30 g |
|
L-lysine |
5.80 g |
|
(As lysine hydrochloride) |
(7.25 g) |
|
L-methionine |
4.00 g |
|
L-phenylalanine |
5.60 g |
|
L-proline |
6.80 g |
|
L-serine |
5.00 g |
|
L-threonine |
4.20 g |
|
L-tryptophan |
1.80 g |
|
L-tyrosine |
0.40 g |
|
L-valine |
5.80 g |
|
Glucose |
250.00 g |
|
(As glucose monohydrate) |
(275.00 g) |
|
Total calories (kcal) |
2000 |
|
Non-protein calories (kcal) |
1600 |
* Mixture of refined olive oil (approximately 80%) and refined soya bean oil (approximately 20%).
The other ingredients are purified egg phosphatide, glycerol, sodium oleate, sodium hydroxide, glacial acetic acid, hydrochloric acid, water for injections.
What OLICLINOMEL N8-800, emulsion for infusion looks like and contents of the pack
OLICLINOMEL N8-800 is an emulsion for infusion package in a 3-chamber bag, which is a multi-layer plastic bag. The inner (contact) layer of the bag material is made from polymers (blend of polyolefinic copolymers) compatible with constituent (amino acid solutions, glucose solutions and lipid emulsions) and authorized additives and allowing to make peelable seals. The polymers used for the entire film structure are made of EVA (poly(ethylene-vinyl acetate)), and of a copolyester.
Before the contents of the 3 chambers in the bag are mixed, 1 chamber contains a homogenous liquid with a milky appearance (the lipid emulsion), while the two other chambers (containing the amino acid solution and the glucose solution) contain a colourless or lightly yellow solution, practically free of visible particles. Once mixed, OLICLINOMEL N8-800 is an emulsion for infusion which looks like a homogenous and milky-white liquid.
To prevent contact with oxygen from the air the bag is packaged in an oxygen barrier overpouch, which contains an oxygen absorber sachet.
Packsizes 2000 ml bag: carton with 4 bags
Marketing Authorisation Holder
For any information about OLICLINOMEL, please contact the Marketing Authorisation
Holder: Baxter Healthcare Limited
Caxton Way
Thetford
Norfolk IP24
3SE United
Kingdom
Manufacturer
Baxter S.A., Boulevard Rene Branquart, 80, 7860 Lessines, Belgium
This medicinal product is authorised in the Member States of the EEA under the following names: Oliclinomel N8-800
In some countries it is registered under a different trade name, as described below:
Austria: Oliclinomel 5% GF
Cyprus and Greece: Multimel N8-800
Italy: Acumel
This leaflet was last revised in 11/2015
Baxter and Oliclinomel are trademarks of Baxter International Inc.
The following information is intended for healthcare professionals only:
OLICLINOMEL N8-800, Emulsion for Infusion
1. Quantitative composition
After the contents of the 3 compartments have been mixed, the appearance of the mixture is a homogenous milk-like emulsion. The mixed emulsion for each of the different bag presentations provides the following:
|
Per bag |
2000 ml |
|
Nitrogen (g) |
16.5 |
|
Amino acids (g) |
100 |
|
Glucose (g) |
250 |
|
Lipids (g) |
60 |
|
Total calories (kcal) |
2000 |
|
Non-protein calories (kcal) |
1600 |
|
Glucose calories (kcal) |
1000 |
|
Lipid calories (kcal) |
600 |
|
Non-protein calorie/nitrogen ratio (kcal/g N) |
97 |
|
Phosphate (mmol)** |
4.5 |
|
Acetate (mmol) |
85 |
|
Chloride (mmol) |
40 |
** Phosphates provided by the lipid emulsion
2. Posology and method of administration
The dosage depends on the patient’s energy expenditure, clinical status, body weight, and the ability to metabolize the constituents of OLICLINOMEL N8-800, as well as additional energy or proteins provided orally/enterally; therefore, the bag size should be chosen accordingly.
The administration may be continued for as long as is required by the patient’s clinical conditions.
The maximum daily dose should not be exceeded in adult and pediatric patients. Due to the static composition of the multi-chamber bag, the ability to simultaneously meet all nutrient needs of the patient may not be possible. Clinical situations may exist where patients require amounts of nutrients varying from the composition of the static bag.
OLICLINOMEL N8-800 is for single use only.
The recommended duration of the parenteral nutrition infusion is between 12 and 24 hours.
Dosage and infusion rate - Adults
Average nitrogen requirements are 0.16 to 0.35 g/kg/day (approximately 1 to 2 g amino acids/kg/day). Energy requirements vary depending on the patient's nutritional state and level of catabolism. On average these are 20 to 40 kcal/kg/day.
Maximum daily dose:
The maximum daily dose is 40 ml/kg body weight (equivalent to 2.00 g amino acids, 5.00 g glucose and 1.20 g lipids/kg), i.e. 2800 ml of the emulsion for infusion for a patient weighing 70 kg.
Maximum infusion rate:
As a general rule, do not exceed 2 ml/kg/hour of the emulsion for infusion, i.e. 0.10 g amino acids, 0.25 g glucose and 0.06 g lipids / kg body weight / hour.
There have been no studies performed in the paediatric population.
Average nitrogen requirements are:
Children 2-11 years of age: 0.16 to 0.35 (up to 0.45) g/kg/day equivalent to 1-2 (up to 3) g amino acids/kg/day.
Children 12-18 years of age: 0.16 to 0.35 g/kg/day equivalent to 1- 2 g amino acids/kg/day.
Energy requirements vary depending on the patient's age, nutritional state and level of catabolism. On average these range between:
Children 2-11 years of age: 60 - 90 kcal/kg/day Children 12-18 years of age: 30 -75 kcal/kg/day
The dosage is based on fluid intake and daily nitrogen requirements.
These intakes should be adjusted to take account of the child's hydration status.
Maximum daily dose:
The maximum daily dose is 60 ml/kg body weight (equivalent to 3 g amino acids, 7.5 g glucose and 1.8 g lipids/kg body weight).
Maximum infusion rate in children 2-11 years of age:
In this group of age, the limiting factor for hourly rate is the amino acid concentration. As a general rule, do not exceed 4 ml/kg/hour of the emulsion for infusion, i.e. 0.20 g amino acids, 0.5 g glucose and 0.12 g lipids/kg body weight per hour.
Maximum infusion rate in children 12-18 years of age:
In this group of age, the limiting factor for hourly rate is the amino acid concentration. As a general rule, do not exceed 2.4 ml/kg/hour of the emulsion for infusion, i.e. 0.12 g amino acids, 0.3 g glucose and 0.07 g lipids/kg body weight per hour.
Route of administration
OLICLINOMEL N8-800 must be administered intravenously through a central vein.
The administration flow rate should be adjusted to take account of the dose being administered, the characteristics of the final mixture being infused, the daily volume intake and the duration of the infusion.
3. Special warnings and precautions for use
• Do not administer through a peripheral vein.
• An excessively fast administration of total parenteral nutrition (TPN) solutions, including OLICLINOMEL, may result in severe or fatal consequences.
• The infusion must be stopped immediately if any abnormal signs or symptoms of an allergic reaction (such as sweating, fever, chills, headache, skin rashes, dyspnoea or bronchospasm) develop. This medicinal product contains soybean oil and egg phosphatide. Soybean and egg proteins may cause hypersensitivity reactions. Cross-allergic reactions between soybean and peanut proteins have been observed.
• Severe water and electrolyte equilibration disorders, severe fluid overload states, and severe metabolic disorders must be corrected before starting the infusion.
• Specific clinical monitoring is required when an intravenous infusion is started.
• Pulmonary vascular precipitates causing pulmonary vascular embolism and respiratory distress have been reported in patients receiving parenteral nutrition. In some cases, fatal outcomes have occurred. Excessive addition of calcium and phosphate increases the risk of formation of calcium phosphate precipitates (see section 4, “Incompatibilities”). Precipitates of various natures have been reported even in the absence of phosphate salt in the solution. Suspected precipitate formation in the blood stream has also been reported.
In addition to inspection of the solution, the infusion set and catheter should also periodically be checked for precipitates.
If signs of respiratory distress occur, the infusion should be stopped and medical evaluation initiated.
• Do not add other medicinal products or substances to any components of the bag or to the reconstituted emulsion without first confirming their compatibility and the stability of the resulting preparation (in particular the stability of the lipid emulsion). Formation of precipitates or destabilization of the lipid emulsion could result in vascular occlusion.
• Vascular access infection and sepsis are complications that may occur in patients receiving parenteral nutrition, particularly in case of poor maintenance of catheters, immunosuppressive effects of illness or drugs. Careful monitoring of signs, symptoms, and laboratory tests for fever/chills, leukocytosis, technical complications with the access device and hyperglycaemia can help recognize early infections. Patients who require parenteral nutrition are often predisposed to infectious complications due to malnutrition and/or their underlying disease state. The occurrence of septic complications can be decreased with heightened emphasis on aseptic techniques in catheter placement, maintenance, as well as aseptic techniques in the preparation of the nutritional formula
• Monitor water and electrolyte balance, serum osmolarity, serum triglycerides, acid-base balance, blood glucose, liver and kidney function, and blood count, including platelets and coagulation parameters throughout treatment.
• Metabolic complications may occur if the nutrient intake is not adapted to the patient's requirements, or the metabolic capacity of any given dietary component is not accurately assessed. Adverse metabolic effects may arise from administration of inadequate or excessive nutrients or from inappropriate composition of an admixture for a particular patient's needs.
• Serum triglyceride concentrations and the ability of the body to remove lipids must be checked regularly.
• Serum triglyceride concentrations must not exceed 3 mmol/l during the infusion. These concentrations should not be determined before a minimum of a 3-hour period of continuous infusion.
• If a lipid metabolism abnormality is suspected, it is recommended that tests be performed daily by measuring serum triglycerides after a period of 5 to 6 hours without administering lipids. In adults, the serum must be clear in less than 6 hours after stopping the infusion containing the lipid emulsion. The next infusion should only be administered when the serum triglyceride concentrations have returned to normal values.
• Fat overload syndrome has been reported with similar products. The reduced or limited ability to metabolize the lipids contained in OLICLINOMEL may result in a "fat overload syndrome" which may be caused by overdose; however the signs and symptoms of this syndrome may also occur when the product is administered according to instructions.
• In the event of hyperglycaemia, the infusion rate of OLICLINOMEL N8-800 must be adjusted and/or insulin administered.
• When making additions, the final osmolarity of the mixture must be measured before administration. The mixture obtained should be administered through a central or peripheral venous line depending on its final osmolarity. If the final mixture administered is hypertonic it
may cause irritation of the vein when administered into a peripheral vein.
• Although there is a natural content of trace elements and vitamins in the product, the levels are insufficient to meet body requirements and these should be added to prevent deficiencies from developing. See instructions for making additions to this product.
• Caution should be exercised in administering OLICLINOMEL N8-800 to patients with increased osmolarity, adrenal insufficiency, heart failure or pulmonary dysfunction.
• Refeeding severely undernourished patients may result in the refeeding syndrome that is characterized by the shift of potassium, phosphorus, and magnesium intracellularly as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. Careful monitoring and slowly increasing nutrient intakes while avoiding overfeeding can prevent these complications. This syndrome has been reported with similar products.
• Do not connect bags in series in order to avoid air embolism due to residual gas contained in the primary bag.
Hepatic Insufficiency
Use with caution in patients with hepatic insufficiency because of the risk of developing or worsening neurological disorders associated with hyperammonaemia. Regular clinical and laboratory tests are required particularly controlling liver function parameters, blood glucose, electrolytes and triglycerides.
Renal Insufficiency
Use with caution in patients with renal insufficiency, particularly if hyperkalaemia is present, because of the risk of developing or worsening metabolic acidosis and hyperazotemia if extra-renal waste removal is not being performed. Fluid triglycerides and electrolyte status should be closely monitored in these patients.
Hematologic
Use with caution in patients with coagulation disorders and anaemia. Blood count and coagulation parameters should be closely monitored.
Endocrine and Metabolism Use with caution in patients with:
• Metabolic acidosis. Administration of carbohydrates is not recommended in the presence of lactic acidosis. Regular clinical and laboratory tests are required.
• Diabetes mellitus. Monitor glucose concentrations, glucosuria, ketonuria and, where applicable adjust insulin dosages.
• Hyperlipidaemia due to the presence of lipids in the emulsion for infusion. Regular clinical and laboratory tests are required.
• Amino acid metabolism disorders
Extravasation
Catheter site should be monitored regularly to identify signs of extravasation. If extravasation occurs, the administration should be stopped immediately, keeping the inserted catheter or cannula in place for immediate management of the patient. If possible, aspiration should be performed through the inserted catheter/cannula, in order to reduce the amount of fluid present in the tissues before removing the catheter/cannula. When involving an extremity, the concerned limb should be elevated.
Depending on the extravasated product (including the product(s) being mixed with OLICLINOMEL N8-800, if applicable) and the stage/extent of any injury, appropriate specific measures should be taken. Options for management may include non-pharmacologic, pharmacologic and/or surgical intervention. If there is any deterioration of the affected area (continued pain, necrosis, ulceration, suspected compartment syndrome), surgery should be consulted immediately.
The extravasation site should be monitored at least every 4 hours during the 24 first hours, then once daily.
The infusion should not be restarted in the same peripheral or central vein.
Special precautions in paediatrics
When administered to children greater than 2 years old, it is essential to use a bag which has a volume corresponding to the daily dosage.
Vitamin and trace element supplementation is always required. Paediatric formulations should be used.
4. Practical information on preparation and handling
Only use OLICLINOMEL N8-800 if:
- the bag is undamaged,
- the non-permanent seals are intact,
- the glucose and amino acid solutions are clear, colourless or slightly yellow, practically free of visible particles,
- the lipid emulsion is homogeneous and milk-like.
OLICLINOMEL N8-800 should be at room temperature before use.
Only administer the product after the non-permanent seals between the 3 compartments have been broken and the contents of the 3 compartments have been mixed as shown below.
Ensure that the final emulsion for infusion does not show any evidence of phase separation.

Tear from the top to open the overpouch.
2.

Peel the front of the overpouch to reveal the OLICLINOMEL N8-800 bag. Discard the overpouch and oxygen absorber sachet.
Place the bag flat on a horizontal and clean surface with handle in front of you.
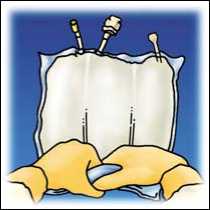
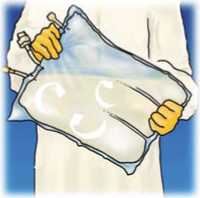
Lift the hanger area to remove solution from the upper bag. Firmly roll the upper bag until peal seal is fully open (approximately half way).
Mix by turning the bag upside-down at least 3 times. Ensure a homogenous mixture, with no evidence of phase separation
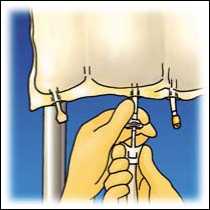
Hang the bag. Twist off the protector from the administration outlet. Firmly plug the spike connector.
After opening the bag, the content must be used immediately. The opened bag must never be stored for a subsequent infusion.
Do not reconnect any partially used bag.
Do not connect bags in series in order to avoid the possibility of air embolism due to gas contained in the primary bag.
For single use only. Any unused product or waste material and all necessary devices must be discarded.
Do not store any partially used bags and discard all devices after use.
Supplementation
Do not add other medicinal products or substances to any components of the bag or to the reconstituted emulsion without first confirming their compatibility and the stability of the resulting preparation (in particular stability of the lipid emulsion).
However, OLICLINOMEL N8-800 can be used as such or after supplementation with electrolytes, trace elements or vitamins, when required.
The capacity of the bag is sufficient to enable additions such as, vitamins, electrolytes, and trace elements. Any additions (including vitamins) may be made into the reconstituted mixture (after the non-permanent seals have been opened and the contents of the three compartments have been mixed).
Vitamins may also be added into the glucose compartment before the mixture has been reconstituted (before opening the non-permanent seals and before mixing the solutions and the emulsion).
When making additions to the formulation, the final osmolarity of the mixture should be measured before administration via a peripheral vein.
OLICLINOMEL N8-800 may be supplemented with:
• Electrolytes: stability has been demonstrated up to a total quantity of 150 mmol of sodium, 150 mmol of potassium, 5.6 mmol of magnesium and 5 mmol of calcium per litre of the ternary mixture.
• Organic phosphate: stability has been demonstrated for additions of up to 22 mmol per litre.
Trace elements and vitamins: Stability has been demonstrated with commercially available preparations of vitamins and trace elements (containing up to 1 mg of iron). Compatibility for other additives is available upon request.
Additions must be performed by qualified personnel under aseptic conditions.
These additions are made into the injection site using a needle:
- Prepare the injection site,
- Puncture the injection site and inject,
- Mix the contents of the bag and the additives.
Interactions
No interaction studies have been performed with OLICLINOMEL N8-800.
OLICLINOMEL N8-800 contains vitamin K, naturally present in lipid emulsions. The amount of Vitamin K in recommended doses of OLICLINOMEL N8-800 are not expected to influence effects of coumarin derivatives.
The lipids contained in this emulsion may interfere with the results of certain laboratory tests (for example, bilirubin, lactate dehydrogenase, oxygen saturation, blood haemoglobin) if the blood sample is taken before the lipids have been eliminated (these are generally eliminated after a period of 5 to 6 hours without receiving lipids).
Incompatibilities
This emulsion for infusion must not be administered simultaneously with blood through the same infusion tubing.
Incompatibilities may be produced for example by excessive acidity (low pH) or inappropriate content of divalent cations (Ca2+ and Mg2+), which may de-stabilise the lipid emulsion.
As with any parenteral nutrition admixture, calcium and phosphate ratios must be considered. Excess addition of calcium and phosphate, especially in the form of mineral salts, may result in formation of calcium phosphate precipitates.
Check compatibility with solutions administered simultaneously through the same giving set, catheter or cannula.
5. Shelf life 2 years if the overwrap is not damaged.
It is recommended that the product is used immediately after the non-permanent seals between the 3 compartments have been opened. The reconstituted emulsion has, however, been shown to be stable for a maximum of 7 days at between +2o and +8oC followed by a maximum of 48 h at temperatures not exceeding + 25oC.
After supplementation (electrolytes, trace elements, vitamins) of reconstituted OLICLINOMEL N8-800, (see previous section), chemical and physical in-use stability has been demonstrated for 7 days at 2 to 8°C followed by 48 hours below 25°C. From a microbiological point of view, any admixture should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless addition of supplements has taken place in controlled and validated aseptic conditions.
Baxter and Oliclinomel are trademarks of Baxter International Inc.