Omeflex Peri Emulsion For Infusion
Package leaflet: Information for the user
Omeflex peri emulsion for infusion
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Omeflex peri is and what it is used for
2. What you need to know before you use Omeflex peri
3. How to use Omeflex peri
4. Possible side effects
5. How to store Omeflex peri
6. Contents of the pack and other information
1. What Omeflex peri is and what it is used for
Omeflex peri contains fluids and substances called amino acids, electrolytes and fatty acids that are essential for the body to grow or to recover. It also contains calories in the form of carbohydrates and fats.
Omeflex peri is given to adults.
You are given Omeflex peri when you are unable to eat food normally. There are many situations when this might be the case, for example when you are recovering from surgery, injuries, or burns, or when you are unable to absorb food from your stomach and gut.
2. What you need to know before you use Omeflex peri Do not use Omeflex peri
• if you are allergic to any of the active substances, to egg, peanut, soybean or fish or to any of the other ingredients of this medicine (listed in section 6).
• This medicine must not be given to newborn infants, infants and toddlers under two years old.
Also, do not use Omeflex peri if you suffer from any of the following:
• life-threatening blood circulation problems such as those that can occur if you are in a state of collapse or shock
• heart attack or stroke
• severely impaired blood clotting function , bleeding risk (severe coagulopathy, aggravating haemorrhagic
diatheses)
• blocking of blood vessels by blood clots or fat (embolism)
• severe liver failure
• impaired bile flow (intrahepatic cholestasis)
• severe kidney failure in the absence of kidney replacement therapy
• disturbances of your body salt composition
• fluid deficit or excess water in your body
• water on your lungs (pulmonary oedema)
• severe heart failure
• certain metabolic disorders such as
- too much lipid (fat) in the blood
- inborn errors of amino acid metabolism
- abnormally high blood sugar level that needs more than 6 units of insulin per hour to be controlled
- abnormalities of metabolism that may occur after operations or injuries
- coma of unknown origin
- insufficient supply of oxygen to tissues
- abnormally high acid level in the blood.
Warnings and precautions
Talk to your doctor before using Omeflex peri.
Please inform your doctor if:
• you have heart, liver or kidney problems
• you suffer from certain types of metabolic disorders such as diabetes, abnormal blood fat values and disorders of your body fluid and salt composition or your acid-base balance
You will be monitored closely to detect early signs of an allergic reaction (such as fever, shivering, rash, or shortness of breath) when you receive this medicine.
Further monitoring and tests such as various examinations of blood samples will be applied to make sure that your body handles the administered foodstuffs properly.
The nursing staff may also take measures to ensure that your body’s fluid and electrolyte requirements are met. In addition to Omeflex peri you may receive further nutrients (foodstuffs) in order to fully cover your requirements.
Children
The safety and efficacy in children over 2years have not been established yet. No data are available.
This medicine must not be given to newborn infants, infants and toddlers under two years old.
Other medicines and Omeflex peri
Tell your doctor if you are taking, have recently taken or might take any other medicines.
Omeflex peri can interact with some other medicines. Please tell your doctor if you are taking or receiving any of the following:
• insulin
• heparin
• medicines that prevent undesirable blood clotting such as warfarin or other coumarin derivatives
• medicines to promote urine flow (diuretics)
• medicines to treat high blood pressure or heart problems (ACE-inhibitors and angiotensin-II-receptor antagonists)
• medicines used in organ transplants such as ciclosporin and tacrolimus
• medicines to treat inflammation (corticosteroids)
• hormone preparations that affect your fluid balance (adrenocorticotropic hormone [ACTH])
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. If you are pregnant you will receive this medicine only if the doctor considers it absolutely necessary for your recovery. There is no data available about the use of Omeflex peri in pregnant women.
Breast-feeding is not recommended for mothers on parenteral nutrition.
Driving and using machines
This medicine is normally given to immobile patients , e.g. in a hospital or clinic which would exclude driving or using machines. However, the medicine itself has no effect on the ability to drive or use machines.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
3. How to use Omeflex peri
This medicine is administered by intravenous infusion (drip), that is, through a small tube directly into a vein. This medicine can be administered through one of your smaller (peripheral) or larger (central) veins. The recommended duration of infusion for a parenteral nutrition bag is maximum 24 h.
Your doctor will decide how much of this medicine you need and for how long you will require treatment with this medicine.
Use in children and adolescents
The safety and efficacy in children over 2years have not been yet established. No data are available.
This medicine must not be given to newborn infants, infants and toddlers under two years old.
If you use more Omeflex peri than you should
If you have received too much of this medicine you may suffer from a so-called ‘overload syndrome’ and the following symptoms:
• fluid excess and electrolyte disorders
• water on your lungs (pulmonary oedema)
• loss of amino acids through the urine and disturbed amino acid balance
• vomiting, feeling sick
• shivering
• high blood sugar level
• glucose in the urine
• fluid deficit
• blood much more concentrated than normal (hyperosmolality)
• impairment or loss of consciousness due to extremely high blood sugar
• enlargement of the liver (hepatomegaly) with or without jaundice (icterus)
• enlargement of the spleen (splenomegaly)
• fat deposition in the inner organs
• abnormal values of liver function tests
• reduction of red blood cell count (anaemia)
• reduction of white blood cell count (leucopenia)
• reduction of blood platelet count (thrombocytopenia)
• increase of immature red blood cells (reticulocytosis)
• rupture of blood cells (haemolysis)
• bleeding or a tendency to bleeding
• impairment of blood coagulation (as can be seen by changes of bleeding time, coagulation time, prothrombin time etc.)
• fever
• high blood fat levels
• loss of consciousness
If any of these symptoms occur, the infusion must be stopped immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may be serious. If any of the following side effects occur, tell your doctor immediately, he will stop giving you this medicine:
Rare (may affect up to in 1,000 people):
• allergic reactions, for example skin reactions, shortness of breath, swelling of the lips, mouth, and throat, difficulty breathing
Other side effects include:
Common (may affect up to 1 in 10 people):
• irritation or inflammation of veins (phlebitis, thrombophlebitis)
Uncommon (may affect up to 1 in 100 people):
• feeling sick, vomiting, loss of appetite
Rare (may affect up to 1 in 1,000 people):
• increased tendency for your blood to clot
• bluish discolouration of the skin
• shortness of breath
• headache
• flushing
• reddening of skin (erythema)
• sweating
• chills
• feeling cold
• high body temperature
• drowsiness
• pain in the chest, back, bones or lumbar region
• decrease or increase in blood pressure
Very rare (may affect up to 1 in 10,000 people):
• abnormally high blood fat or blood sugar values
• high levels of acidic substances in your blood
• Too much lipid can lead to fat overload syndrome, for more information on this please see under the heading “If you use more Omeflex peri than you should” in section 3. Symptoms normally disappear when the infusion is stopped.
Not known (frequency cannot be estimated from the available data):
• reduction of white blood cell count (leucopenia)
• reduction of blood platelet count (thrombocytopenia)
• impaired bile flow (cholestasis)
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom:
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Ireland:
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; e-mail: medsafety@hpra.ie
5. How to store Omeflex peri
Keep this medicine out of the sight and reach of children.
Do not store above 25 °C.
Do not freeze. If accidentally frozen, discard the bag.
Keep the bag in the protective overwrap in order to protect from light.
Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last date of that month.
6. Contents of the pack and other information What Omeflex peri contains
The active substances in the ready-for-use mixture are:
|
from the top chamber (glucose solution) |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Glucose monohydrate equivalent to anhydrous glucose |
70.40 g 64.00 g |
88.00 g 80.00 g |
132.0 g 120.0 g |
176.0 g 160.0 g |
|
Sodium dihydrogen phosphate dihydrate |
0.936 g |
1.170 g |
1.755 g |
2.340 g |
|
Zinc acetate dihydrate |
5.280 mg |
6.600 mg |
9.900 mg |
13.20 mg |
|
from the middle chamber (fat emulsion) |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Medium-chain triglycerides |
20.00 g |
25.00 g |
37.50 g |
50.00 g |
|
Soya-bean oil, refined |
16.00 g |
20.00 g |
30.00 g |
40.00 g |
|
Omega-3-acid triglycerides |
4.000 g |
5.000 g |
7.500 g |
10.00 g |
|
from the bottom chamber (amino acid solution) |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Isoleucine |
1.872 g |
2.340 g |
3.510 g |
4.680 g |
|
Leucine |
2.504 g |
3.130 g |
4.695 g |
6.260 g |
|
Lysine hydrochloride equivalent to lysine |
2.272 g 1.818 g |
2.840 g 2.273 g |
4.260 g 3.410 g |
5.680 g 4.546 g |
|
Methionine |
1.568 g |
1.960 g |
2.940 g |
3.920 g |
|
Phenylalanine |
2.808 g |
3.510 g |
5.265 g |
7.020 g |
|
Threonine |
1.456 g |
1.820 g |
2.730 g |
3.640 g |
|
Tryptophan |
0.456 g |
0.570 g |
0.855 g |
1.140 g |
|
Valine |
2.080 g |
2.600 g |
3.900 g |
5.200 g |
|
Arginine |
2.160 g |
2.700 g |
4.050 g |
5.400 g |
|
Histidine hydrochloride monohydrate equivalent to histidine |
1.352 g 1.000 g |
1.690 g 1.251 g |
2.535 g 1.876 g |
3.380 g 2.502 g |
|
Alanine |
3.880 g |
4.850 g |
7.275 g |
9.700 g |
|
Aspartic acid |
1.200 g |
1.500 g |
2.250 g |
3.000 g |
|
Glutamic acid |
2.800 g |
3.500 g |
5.250 g |
7.000 g |
|
Glycine |
1.320 g |
1.650 g |
2.475 g |
3.300 g |
|
Proline |
2.720 g |
3.400 g |
5.100 g |
6.800 g |
|
Serine |
2.400 g |
3.000 g |
4.500 g |
6.000 g |
|
Sodium hydroxide |
0.640 g |
0.800 g |
1.200 g |
1.600 g |
|
Sodium chloride |
0.865 g |
1.081 g |
1.622 g |
2.162 g |
|
Sodium acetate trihydrate |
0.435 g |
0.544 g |
0.816 g |
1.088 g |
|
Potassium acetate |
2.354 g |
2.943 g |
4.415 g |
5.886 g |
|
Magnesium acetate tetrahydrate |
0.515 g |
0.644 g |
0.966 g |
1.288 g |
|
Calcium chloride dihydrate |
0.353 g |
0.441 g |
0.662 g |
0.882 g |
|
Electrolytes |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Sodium |
40 mmol |
50 mmol |
75 mmol |
100 mmol |
|
Potassium |
24 mmol |
30 mmol |
45 mmol |
60 mmol |
|
Magnesium |
2.4 mmol |
3.0 mmol |
4.5 mmol |
6.0 mmol |
|
Calcium |
2.4 mmol |
3.0 mmol |
4.5 mmol |
6.0 mmol |
|
Zinc |
0.024 mmol |
0.03 mmol |
0.045 mmol |
0.06 mmol |
|
Chloride |
38 mmol |
48 mmol |
72 mmol |
96 mmol |
|
Acetate |
32 mmol |
40 mmol |
60 mmol |
80 mmol |
|
Phosphate |
6.0 mmol |
7.5 mmol |
11.25 mmol |
15.0 mmol |
|
Amino acid content |
32 g |
40 g |
60 g |
80 g |
|
Nitrogen content |
4.6 g |
5.7 g |
8.6 g |
11.4 g |
|
Carbohydrate content |
64 g |
80 g |
120 g |
160 g |
|
Lipid content |
40 g |
50 g |
_75g |
100 g |
|
Energy in the form of lipids |
1590 kJ (380 kcal) |
1990 kJ (475 kcal) |
2985 kJ (715 kcal) |
3980 kJ (950 kcal) |
|
Energy in the form of carbohydrates |
1075 kJ (255 kcal) |
1340 kJ (320 kcal) |
2010 kJ (480 kcal) |
2680 kJ (640 kcal) |
|
Energy in the form of amino acids |
535 kJ (130 kcal) |
670 kJ (160 kcal) |
1005 kJ (240 kcal) |
1340 kJ (320 kcal) |
|
Non-protein energy |
2665 kJ (635 kcal) |
3330 kJ (795 kcal) |
4995 kJ (1195 kcal) |
6660 kJ (1590 kcal) |
|
Total energy |
3200 kJ (765 kcal) |
4000 kJ (955 kcal) |
6000 kJ (1435 kcal) |
8000 kJ (1910 kcal) |
|
Osmolality |
950 mOsm/ kg |
950 mOsm/ kg |
950 mOsm/kg |
950 mOsm/ kg |
|
Theoretical osmolarity |
840 mOsm/l |
840 mOsm/l |
840 mOsm/l |
840 mOsm/l |
|
pH |
5.0 - 6.0 |
5.0 - 6.0 |
5.0 - 6.0 |
5.0 - 6.0 |
The other ingredients are citric acid monohydrate (for pH adjustment), egg lecithin, glycerol, sodium oleate, all-rac-alpha-tocopherol, sodium hydroxide (for pH adjustment) and water for injections.
What Omeflex peri looks like and contents of the pack
The ready-to-use product is an emulsion for infusion, i.e. it is administered through a small tube into a vein. Omeflex peri is supplied in flexible multichamber bags containing:
• 1250 ml (500 ml of amino acids solution + 250 ml of fat emulsion + 500 ml of glucose solution)
• 1875 ml (750 ml of amino acids solution + 375 ml of fat emulsion + 750 ml of glucose solution)
• 2500 ml (1000 ml of amino acids solution + 500 ml of fat emulsion + 1000 ml of glucose solution)
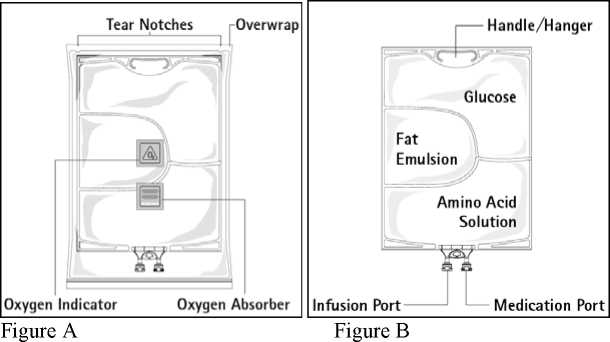
Figure A: The multichamber bag is packed in a protective overwrap. An oxygen absorber and an oxygen indicator are placed between the bag and the overwrap; the oxygen absorber sachet is made of inert material and contains iron hydroxide.
Figure B: The top chamber contains a glucose solution, the middle chamber contains a fat emulsion, and the bottom chamber contains an amino acid solution.
The glucose and the amino acid solutions are clear and colourless up to straw-coloured. The fat emulsion is milky-white.
The top chamber and the middle chamber can be connected with the bottom chamber by opening the intermediate seams.
The different container sizes are presented in cartons containing five bags.
Pack sizes: 5 x 1250 ml, 5 x 1875 ml and 5 x 2500 ml Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer:
B. Braun Melsungen AG
Carl-Braun-Strabe 1 Postal address:
34212 Melsungen, Germany 34209 Melsungen, Germany
Phone: +49-5661-71-0 Fax: +49-5661-71-4567
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria
Belgium
Bulgaria
Croatia
Cyprus
Czech Republic
Denmark
Estonia
Finland
France
Germany
Greece
Hungary
Ireland
Italy
Lithuania
Luxembourg
Latvia
Netherlands
Norway
NuTRIflex Omega peri novo
Nutriflex Omega peri emulsie voor infusie
HyTPI/l^neKC OMera nepu
Nutriflex Omega peri
Nutriflex Omega peri
Nutriflex Omega peri
Omegaflex peri
Nutriflex Omega peri
Nutriflex Omega 32/64/40 perifer
Perinutriflex Omega E, emulsion pour perfusion
NuTRIflex Omega peri novo
Nutriflex Omega peri
Nutriflex Omega peri
Omeflex peri emulsion for infusion
Nutriplus Omega AA32/G64
Nutriflex Omega peri
NuTRIflex Omega peri novo
Nutriflex Omega peri emulsija infuzijam
Nutriflex Omega peri
Omegaflex peri
Nutriflex Omega peri
Poland
Portugal
Romania
Slovakia
Slovenia
Spain
Sweden
United Kingdom
Nutriflex Omega peri
Nutriflex Omega peri emulsie perfuzabila
Nutriflex Omega peri
Nutriflex Omega peri emulzija za infundiranje Nutriflex Omega peri Emulsion para perfusion Nutriflex Omega peri Omeflex peri emulsion for infusion
This leaflet was last revised in 13 June 2016
The following information is intended for healthcare professionals only:
No special requirements for disposal.
Parenteral nutrition products should be visually inspected for damage, discolouration and emulsion instability before use.
Do not use bags which are damaged; neither overwrap nor the primary bag should be damaged. Use only if the peel seams between the chambers are intact, if amino acid and glucose solutions are clear and colourless up to straw-coloured and the emulsion is a homogenous liquid with milky white appearance. Do not use if the solutions are discoloured or contain particulate matter. Do not use if the emulsion shows signs of phase separation (oil drops, oil layer).
Before opening the overwrap, check the colour of the oxygen indicator (see Figure A). Do not use if the oxygen indicator is pink. Use only if the oxygen indicator is yellow.
Preparation of the mixed emulsion
Strict adherence to aseptic handling principles must be complied with.
To open: Tear overwrap starting from the tear notches (Fig. 1). Remove the bag from its protective overwrap. Discard overwrap, oxygen indicator and oxygen absorber.
Visually inspect the primary bag for leaks. Leaky bags must be discarded, since the sterility cannot be guaranteed.
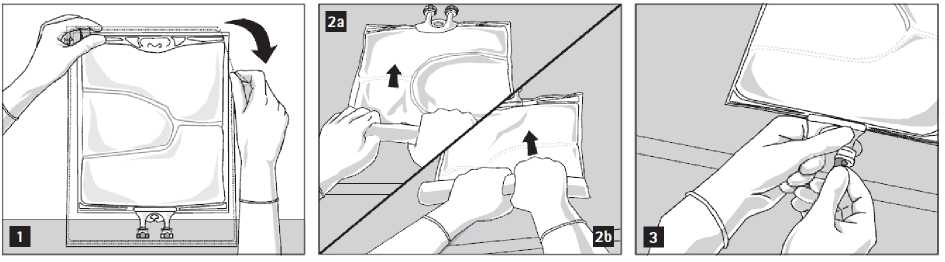
To open and mix the chambers sequentially, roll the bag with both hands, starting first by opening the peel seam that separates the top chamber (glucose) and the bottom chamber (amino acids) (Fig. 2a). Then continue applying pressure so that the peel seam separating the middle chamber (lipids) and the bottom chamber opens (Fig. 2b).
Addition of additives
After removing the aluminium seal (Fig. 3) one can add compatible additives via the medication port (Fig. 4).
Omeflex peri can be mixed with following additives:
N(2)-L-alanyl-L-glutamine Aqua ad injectabilia Calcium gluconate 10%
Sodium chloride 20%
Potassium chloride 14.9%
Magnesium sulphate 50%
Potassium dihydrogenphosphate 1M Tracutil (trace elements)
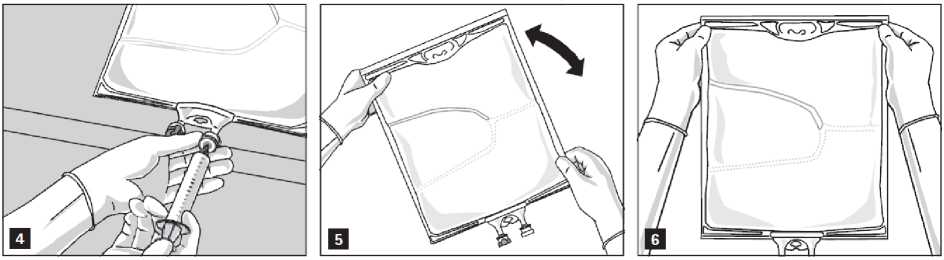
Mix the contents of the bag thoroughly (Fig. 5) and visually inspect the mixture (Fig. 6). There should be no signs of emulsion phase separation.
The mixture is a milky-white homogenous oil-in-water emulsion.
Preparation for infusion
The emulsion should always be brought to room temperature prior to infusion.
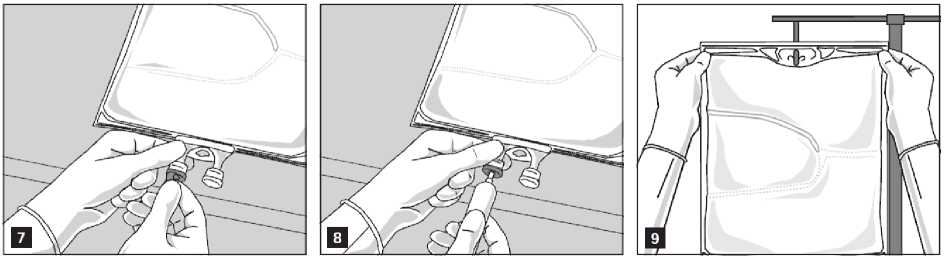
Remove the aluminium foil from the infusion port (Fig. 7) and attach the infusion set (Fig. 8). Use a non-vented infusion set or close the air vent when using a vented set. Hang the bag on an infusion stand (Fig. 9) and carry out infusion using the standard technique.
For single use only. Container and unused residues must be discarded after use.
Do not reconnect partially used containers.
If filters are used they must be lipid-permeable (pore size > 1.2 pm).
Shelf life after removing the protective overwrap and after mixing of the contents of the bag
After mixing the contents of the chambers the final emulsion is to be used immediately. Shelf life after admixture of compatible additives
From a microbiological point of view, the product should be used immediately after admixture of additives. If not used immediately after admixture of additives, in-use storage times and conditions prior to use are the responsibility of the user.
After first opening (spiking of the infusion port)
The emulsion is to be used immediately after opening of the container.
Omeflex peri must not be mixed with other medicinal products for which compatibility has not been documented.
Omeflex peri should not be given simultaneously with blood in the same infusion set due to the risk of pseudoagglutination.