Omeflex Plus Emulsion For Infusion
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Omeflex plus emulsion for infusion
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
The ready-for-use emulsion for intravenous infusion contains after mixing the chamber contents:
|
from the top chamber (glucose solution) |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Glucose monohydrate |
132.0 g |
165.0 g |
247.5 g |
330.0 g |
|
equivalent to anhydrous glucose |
120.0 g |
150.0 g |
225.0 g |
300.0 g |
|
Sodium dihydrogen phosphate dihydrate |
1.872 g |
2.340 g |
3.510 g |
4.680 g |
|
Zinc acetate dihydrate |
5.264 mg |
6.580 mg |
9.870 mg |
13.16 mg |
|
from the middle chamber (fat emulsion) |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Medium-chain triglycerides |
20.00 g |
25.00 g |
37.50 g |
50.00 g |
|
Soya-bean oil, refined |
16.00 g |
20.00 g |
30.00 g |
40.00 g |
|
Omega-3-acid triglycerides |
4.000 g |
5.000 g |
7.500 g |
10.00 g |
|
from the bottom chamber (amino acid solution) |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Isoleucine |
2.256 g |
2.820 g |
4.230 g |
5.640 g |
|
Leucine |
3.008 g |
3.760 g |
5.640 g |
7.520 g |
|
Lysine hydrochloride equivalent to lysine |
2.728 g 2.184 g |
3.410 g 2.729 g |
5.115 g 4.094 g |
6.820 g 5.459 g |
|
Methionine |
1.880 g |
2.350 g |
3.525 g |
4.700 g |
|
Phenylalanine |
3.368 g |
4.210 g |
6.315 g |
8.420 g |
|
Threonine |
1.744 g |
2.180 g |
3.270 g |
4.360 g |
|
Tryptophan |
0.544 g |
0.680 g |
1.020 g |
1.360 g |
|
Valine |
2.496 g |
3.120 g |
4.680 g |
6.240 g |
|
Arginine |
2.592 g |
3.240 g |
4.860 g |
6.480 g |
|
Histidine hydrochloride monohydrate equivalent to histidine |
1.624 g 1.202 g |
2.030 g 1.503 g |
3.045 g 2.254 g |
4.060 g 3.005 g |
|
Alanine |
4.656 g |
5.820 g |
8.730 g |
11.64 g |
|
Aspartic acid |
1.440 g |
1.800 g |
2.700 g |
3.600 g |
|
Glutamic acid |
3.368 g |
4.210 g |
6.315 g |
8.420 g |
|
Glycine |
1.584 g |
1.980 g |
2.970 g |
3.960 g |
|
Proline |
3.264 g |
4.080 g |
6.120 g |
8.160 g |
|
Serine |
2.880 g |
3.600 g |
5.400 g |
7.200 g |
|
Sodium hydroxide |
0.781 g |
0.976 g |
1.464 g |
1.952 g |
|
Sodium chloride |
0.402 g |
0.503 g |
0.755 g |
1.006 g |
|
Sodium acetate trihydrate |
0.222 g |
0.277 g |
0.416 g |
0.554 g |
|
Potassium acetate |
2.747 g |
3.434 g |
5.151 g |
6.868 g |
|
Magnesium acetate tetrahydrate |
0.686 g |
0.858 g |
1.287 g |
1.716 g |
|
Calcium chloride dihydrate |
0.470 g |
0.588 g |
0.882 g |
1.176 g |
|
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml | |
|
Amino acid content [g] |
38 |
48 |
72 |
96 |
|
Nitrogen content [g] |
5.4 |
6.8 |
10.2 |
13.7 |
|
Carbohydrate content [g] |
120 |
150 |
225 |
300 |
|
Lipid content [g] |
40 |
50 |
75 |
100 |
|
Electrolytes [mmol] |
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml |
|
Sodium |
40 |
50 |
75 |
100 |
|
Potassium |
28 |
35 |
52.5 |
70 |
|
Magnesium |
3.2 |
4.0 |
6.0 |
8.0 |
|
Calcium |
3.2 |
4.0 |
6.0 |
8.0 |
|
Zinc |
0.024 |
0.03 |
0.045 |
0.06 |
|
Chloride |
36 |
45 |
67.5 |
90 |
|
Acetate |
36 |
45 |
67.5 |
90 |
|
Phosphate |
12 |
15 |
22.5 |
30 |
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Emulsion for infusion
Amino acids and glucose solutions: clear, colourless up to straw-coloured solutions Fat emulsion: oil-in-water emulsion, milky white
|
in 1000 ml |
in 1250 ml |
in 1875 ml |
in 2500 ml | |
|
Energy in the form of lipids [kJ (kcal)] |
1590 (380) |
1990 (475) |
2985 (715) |
3980 (950) |
|
Energy in the form of carbohydrates [kJ (kcal)] |
2010 (480) |
2510 (600) |
3765 (900) |
5020 (1200) |
|
Energy in the form of amino acids [kJ (kcal)] |
635 (150) |
800 (190) |
1200 (285) |
1600 (380) |
|
Non-protein energy [kJ (kcal)] |
3600 (860) |
4500 (1075) |
6750 (1615) |
9000 (2155) |
|
Total energy [kJ (kcal)] |
4235 (1010) |
5300 (1265) |
7950 (1900) |
10600 (2530) |
|
Osmolality [mOsm/kg] |
1540 |
|
Theoretical osmolarity [mOsm/l] |
1215 |
|
pH |
5.0 - 6.0 |
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Supply of energy, essential fatty acids including omega-3 and omega-6 fatty acids, amino acids, electrolytes and fluids for parenteral nutrition of patients in states of moderate to severe catabolism when oral or enteral nutrition is impossible, insufficient or contraindicated.
Omeflex plus is indicated in adults.
4.2 Posology and method of administration
Posology
The dosage should be adapted to the patients’ individual requirements.
It is recommended that Omeflex plus be administered continuously. A stepwise increase of the infusion rate over the first 30 minutes up to the desired infusion rate avoids possible complications.
Adults
The maximum daily dose amounts to 40 ml/ kg body weight, corresponding to 1.54 g amino acids /kg body weight per day 4.8 g glucose /kg body weight per day 1.6 g lipid /kg body weight per day.
The maximum rate of infusion is 2.0 ml /kg body weight per hour, corresponding to 0.08 g amino acids /kg body weight per hour
0.24 g glucose /kg body weight per hour 0.08 g lipid /kg body weight per hour.
For a patient weighing 70 kg this corresponds to a maximum infusion rate of 140 ml per hour. The amount of substrate administered is then 5.4 g of amino acids per hour, 16.8 g of glucose per hour and 5.6 g of lipids per hour.
Paediatric population
Omeflex plus is contraindicated in newborn infants, infants and toddlers < 2 years of age (see section 4.3).
The safety and efficacy in children >2years have not been established yet. No data are available.
Patients with renal/hepatic impairment
The doses should be adjusted individually in patients with hepatic or renal insufficiency (see also section 4.4).
Duration of treatment
The duration of treatment for the indications stated is not limited. During the administration of Omeflex plus it is necessary to provide an appropriate amount of trace elements and vitamins.
Duration of infusion of one single bag
The recommended duration of infusion for a parenteral nutrition bag is maximum 24 h.
Method of administration
Intravenous use. For central venous infusion only.
4.3 Contraindications
• hypersensitivity to the active substances, to egg, fish, peanut or soya protein or to any of the excipients listed in section 6.1
• inborn errors of amino acid metabolism
• severe hyperlipidaemia characterized by hypertriglyceridaemia (> 1000 mg/dl or 11.4 mmol/l)
• severe coagulopathy
• hyperglycaemia not responding to insulin doses of up to 6 units insulin/hour
• acidosis
• intrahepatic cholestasis
• severe hepatic insufficiency
• severe renal insufficiency in absence of renal replacement therapy
• aggravating haemorrhagic diatheses
• acute thrombo-embolic events, lipid embolism
On account of its composition Omeflex plus must not be used in newborn infants, infants and toddlers under 2 years of age.
General contraindications to parenteral nutrition include:
• unstable circulatory status with vital threat (states of collapse and shock)
• acute phases of cardiac infarction and stroke
• unstable metabolic condition (e.g. severe postaggression syndrome, coma of unknown origin)
• inadequate cellular oxygen supply
• disturbances of the electrolyte and fluid balance
• acute pulmonary oedema
• decompensated cardiac insufficiency.
4.4 Special warnings and precautions for use
Caution should be exercised in cases of increased serum osmolarity.
Disturbances of the fluid, electrolyte or acid-base balance must be corrected before the start of infusion.
Too rapid infusion can lead to fluid overload with pathological serum electrolyte concentrations, hyperhydration and pulmonary oedema.
Any sign or symptom of anaphylactic reaction (such as fever, shivering, rash or dyspnoea) should lead to immediate interruption of the infusion.
The serum triglyceride concentration should be monitored when infusing Omeflex plus .
Depending on the patient’s metabolic condition, occasional hypertriglyceridaemia may occur. If the plasma triglyceride concentration exceeds 4.6 mmol/l (400 mg/dl) during administration of lipids, it is recommended to reduce the infusion rate. The infusion must be interrupted if the plasma triglyceride concentration exceeds
11.4 mmol/l (1000 mg/dl), as these levels have been associated with acute pancreatitis.
Patients with impaired lipid metabolism
Omeflex plus should be administered cautiously to patients with disturbances of lipid metabolism with increased serum triglycerides, e.g. renal insufficiency, diabetes mellitus, pancreatitis, impaired hepatic function, hypothyroidism (with hypertriglyceridaemia), sepsis, and metabolic syndrome. If Omeflex plus is given to patients with these conditions, more frequent monitoring of serum triglycerides is necessary to assure triglyceride elimination and stable triglyceride levels below 11.4 mmol/l (1000 mg/dl).
In combined hyperlipidaemias and in metabolic syndrome, triglyceride levels react to glucose, lipids and overnutrition. Adjust dose accordingly. Assess and monitor other lipid and glucose sources, and drugs interfering with their metabolism.
The presence of hypertriglyceridaemia 12 hours after lipid administration also indicates a disturbance of lipid metabolism.
Like all solutions containing carbohydrates, the administration of Omeflex plus can lead to hyperglycaemia. The blood glucose level should be monitored. If there is hyperglycaemia, the rate of infusion should be reduced or insulin should be administered. If the patient is receiving other intravenous glucose solutions concurrently, the amount of additionally administered glucose has to be taken into account.
An interruption of administration of the emulsion may be indicated if the blood glucose concentration rises to above 14 mmol/l (250 mg/dl) during administration.
Refeeding or repletion of malnourished or depleted patients may cause hypokalaemia, hypophosphataemia and hypomagnesaemia. Close monitoring of serum electrolytes is mandatory. Adequate supplementation of electrolytes according to deviations from normal values is necessary.
Controls of the serum electrolytes, the water balance, the acid-base balance, and of blood cell counts, coagulation status, hepatic and renal function are necessary.
Substitution of electrolytes, vitamins and trace elements may be necessary as required. As Omeflex plus contains zinc, magnesium, calcium and phosphate, care should be taken when it is co-administered with solutions containing these substances.
Omeflex plus is a preparation of complex composition. It is, therefore, strongly advisable not to add other solutions (as long as compatibility is not proven - see section 6.2).
Omeflex plus should not be given simultaneously with blood in the same infusion set due to the risk of pseudoagglutination (see also section 4.5).
As with all intravenous solutions, especially for parenteral nutrition, strict aseptic precautions are necessary for the infusion of Omeflex plus .
Paediatric population
There is as yet no clinical experience of the use of Omeflex plus in children and adolescents.
Elderly patients
Basically the same dosage as for adults applies, but caution should be exercised in patients suffering from further diseases like cardiac insufficiency or renal insufficiency that may frequently be associated with advanced age.
Patients with diabetes mellitus, impaired cardiac or renal function
Like all large-volume infusion solutions, Omeflex plus should be administered with caution to patients with impaired cardiac or renal function.
There is only limited experience of its use in patients with diabetes mellitus or renal failure.
Interference with laboratory tests
The fat content may interfere with certain laboratory measurements (e.g. bilirubin, lactate dehydrogenase, oxygen saturation) if blood is sampled before fat has been adequately cleared from the blood stream.
4.5 Interaction with other medicinal products and other forms of interaction
Some drugs, like insulin, may interfere with the body’s lipase system. This kind of interaction seems, however, to be of only limited clinical importance.
Heparin given in clinical doses causes a transient release of lipoprotein lipase into the circulation. This may result initially in increased plasma lipolysis followed by a transient decrease in triglyceride clearance.
Soya-bean oil has a natural content of vitamin K. This may interfere with the therapeutic effect of coumarin derivatives which should be closely monitored in patients treated with such drugs.
Potassium-containing solutions like Omeflex plus should be used with caution in patients receiving drugs that increase serum potassium concentration, such as potassium-sparing diuretics (triamterene, amiloride, spironolactone), ACE inhibitors (e.g. captopril, enalapril), angiotensin-II-receptor antagonists (e.g. losartan, valsartan), ciclosporin and tacrolimus.
Corticosteroids and ACTH are associated with sodium and fluid retention.
Omeflex plus should not be given simultaneously with blood in the same infusion set due to the risk of pseudoagglutination (see also section 4.4).
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data from the use of Omeflex plus in pregnant women. Animal studies are insufficient with respect to reproductive toxicity (see section 5.3).
Parenteral nutrition may become necessary during pregnancy. Omeflex plus should only be given to pregnant women after careful consideration.
Breast-feeding
Components/metabolites of Omeflex plus are excreted in human milk, but at therapeutic doses no effects on the breastfed newborns/infants are anticipated. Nevertheless breast-feeding is not recommended for mothers on parenteral nutrition.
Fertility
No data from the use of Omeflex plus available.
4.7 Effects on ability to drive and use machines
Omeflex plus has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Under conditions of correct use, in terms of dosing monitoring, observation of safety restrictions and instructions, undesirable effects may still occur. The following listing includes a number of systemic reactions that may be associated with the use of Omeflex plus .
Undesirable effects are listed according to their frequencies as follows:
Very common
Common
Uncommon
(> 1/10)
(> 1/100 to < 1/10)
(> 1/1,000 to < 1/100)
(> 1/10,000 to < 1/1,000)
Rare
Very rare Not known
(< 1/10,000)
(frequency cannot be estimated from the available data)
Blood and lymphatic system disorders
Rare: Hypercoagulation
Not known: Leucopenia, thrombocytopenia
Immune system disorders
Rare: Allergic reactions (e.g. anaphylactic reactions, dermal eruptions, laryngeal, oral and facial oedema)
Metabolism and nutrition disorders
Very rare: Hyperlipidaemia, hyperglycaemia, metabolic acidosis
The frequency of these undesirable effects is dose-dependent and may be higher under the condition of absolute or relative lipid overdose.
Nervous system disorders Rare: Headache, drowsiness
Vascular disorders
Rare: Hypertension or hypotension, flush
Respiratory, thoracic and mediastinal disorders Rare: Dyspnoea, cyanosis
Gastrointestinal disorders Uncommon: Nausea, vomiting
Metabolism and nutrition disorders Uncommon: Loss of appetite
Hepatobiliary disorders Not known: Cholestasis
Skin and subcutaneous tissue disorders Rare: Erythema, sweating
Musculoskeletal and connective tissue disorders Rare: Pain in the back, bones, chest and lumbar region
General disorders and administration site conditions Rare: Elevated body temperature, feeling cold, chills Very rare: Fat overload syndrome (details see below)
Should adverse reactions occur, the infusion must be stopped.
Should the triglyceride level rise to above 11.4 mmol/l (1000 mg/dl) during infusion, the infusion must be stopped. With levels above 4.6 mmol/l (400 mg/dl), the infusion may be continued at a reduced dosage (see section 4.4).
If the infusion is restarted, the patient should be carefully monitored, especially at the beginning, and serum triglycerides should be determined at short intervals.
Information on particular undesirable effects
Nausea, vomiting and lack of appetite are symptoms often related to conditions for which parenteral nutrition is indicated, and may be associated with parenteral nutrition at the same time.
Fat overload syndrome
Impaired capacity to eliminate triglycerides can lead to “fat overload syndrome” which may be caused by overdose. Possible signs of metabolic overload must be observed. The cause may be genetic (individually different metabolism) or the fat metabolism may be affected by ongoing or previous illnesses. This syndrome may also appear during severe hypertriglyceridaemia, even at the recommended infusion rate, and in association with a sudden change in the patient’s clinical condition, such as renal function impairment or infection. The fat overload syndrome is characterised by hyperlipidaemia, fever, fat infiltration, hepatomegaly with or without icterus, splenomegaly, anaemia, leucopenia, thrombocytopenia, coagulation disorder, haemolysis and reticulocytosis, abnormal liver function tests and coma. The symptoms are usually reversible if the infusion of the fat emulsion is discontinued.
Should signs of a fat overload syndrome occur, the infusion of Omeflex plus should be discontinued immediately.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
Overdose
Symptoms of fluid and electrolyte overdose Hyperhydration, electrolyte imbalance and pulmonary oedema.
Symptoms of amino acid overdose
Renal amino acid losses with consecutive amino acid imbalances, sickness, vomiting and shivering.
Symptoms of glucose overdose
Hyperglycaemia, glucosuria, dehydration, hyperosmolality, hyperglycaemic-hyperosmolar coma.
Symptoms of lipid overdose See section 4.8.
Treatment
Immediate cessation of infusion is indicated for overdose. Further therapeutic measures depend on the particular symptoms and their severity. When infusion is recommenced after the symptoms have declined it is recommended that the infusion rate be raised gradually with monitoring at frequent intervals.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Solutions for parenteral nutrition, combinations ATC code: B 05BA10
Mechanism of action
The purpose of parenteral nutrition is to supply all necessary nutrients and energy for the growth and/or regeneration of tissue as well as for the maintenance of all body functions.
Amino acids are of particular importance, since some of them are essential components for protein synthesis. The simultaneous administration of energy sources (carbohydrates/lipids) is necessary to reserve amino acids for tissue regeneration and anabolism, and prevent their utilisation as energy source.
Glucose is ubiquitously metabolised within the organism. Some tissues and organs, such as CNS, bone marrow, erythrocytes, tubular epithelium, cover their energy requirement exclusively from glucose. In addition glucose acts as a structural building block for various cell substances.
On account of their high energy density lipids are an efficient form of energy supply. Long-chain triglycerides provide the organism with essential fatty acids for the synthesis of cell components. For these purposes the fat emulsion contains medium-chain and long-chain triglycerides (deriving from soya-bean oil and fish oil).
The long-chain triglyceride fraction contains omega-6 and omega-3 triglycerides for supply of polyunsaturated fatty acids. They are primarily intended for the prevention and treatment of essential fatty acid deficiency, but also as a source of energy. Omeflex plus contains essential omega-6 fatty acids, mainly in the form of linoleic acid, and omega-3 fatty acids in the form of alpha-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid. The ratio of omega-6/omega-3 fatty acids in Omeflex plus is approximately 2.5:1.
Medium-chain triglycerides are more rapidly hydrolysed, eliminated from the circulation and completely oxidised than long-chain triglycerides. They are a favoured energy substrate, particularly when there is disturbance of the degradation and/or utilisation of long-chain triglycerides, e.g. when there is a lipoprotein lipase deficiency and/or a deficiency in lipoprotein lipase cofactors.
5.2 Pharmacokinetic properties
Absorption
Omeflex plus is infused intravenously. Hence, all substrates are available for metabolism immediately.
Distribution
The dose, rate of infusion, metabolic situation and individual factors of the patient (level of fasting) are of decisive importance for the maximum triglyceride concentrations reached. When used according to the instructions with due regard to the dosage guidelines the triglyceride concentrations do not, in general, exceed 4.6 mmol/l (400 mg/dl).
Medium-chain fatty acids have a low affinity to albumin. In animal experiments administering pure medium-chain triglyceride emulsions, it has been shown that medium-chain fatty acids can cross the blood-brain barrier, if overdosed. No adverse effects were observed with an emulsion providing a mixture of medium-chain triglycerides and long-chain triglycerides, as long-chain triglycerides have an inhibiting effect on medium-chain triglyceride hydrolysis. Therefore, toxic effects on the brain can be excluded after the administration of Omeflex plus .
Amino acids are incorporated in a variety of proteins in different organs of the body. In addition each amino acid is maintained as free amino acid in the blood and inside cells.
As glucose is water-soluble, it is distributed with the blood over the whole body. At first, the glucose solution is distributed in the intravascular space and then it is taken up into the intracellular space.
No data are available concerning transport of the components through the placental barrier.
Biotransformation
Amino acids that do not enter protein synthesis are metabolised as follows. The amino group is separated from the carbon skeleton by transamination. The carbon chain is either oxidised directly to CO2 or utilised as substrate for gluconeogenesis in the liver. The amino group is also metabolised in the liver to urea.
Glucose is metabolised to CO2 and H2O via the known metabolic routes. Some glucose is utilised for lipid synthesis.
After infusion, triglycerides are hydrolysed to glycerol and fatty acids. Both are incorporated in physiological pathways for energy production, synthesis of biological active molecules, gluconeogenesis and resynthesis of lipids.
In detail, long-chain omega-3 polyunsaturated fatty acids replace arachidonic acid as an eicosanoid substrate in cell membranes and decrease the generation of inflammatory eicosanoids and cytokines in the body. This may be of benefit in patients at risk of developing a hyperinflammatory state and sepsis.
Elimination
Only minor amounts of amino acids are excreted unchanged in urine.
Excess glucose is excreted in urine only if the renal threshold of glucose is reached.
Both the triglycerides of soya-bean oil and medium-chain triglycerides are completely metabolised to CO2 and H2O. Small amounts of lipids are lost only during sloughing of cells from skin and other epithelial membranes. Renal excretion does virtually not
occur.
Preclinical safety data
Non-clinical studies have not been performed with Omeflex plus .
Toxic effects of mixtures of nutrients given as substitution therapy at the recommended dosage are not to be expected.
Reproductive toxicity
Phytoestrogens such as B-sitosterol can be found in various vegetable oils, especially in soya-bean oil. Impairment of fertility was observed in rats and rabbits after subcutaneous and intravaginal administration of B-sitosterol. After administration of pure B-sitosterol a decrease of the testicular weight and a reduction of the sperm concentration in male rats and a lowered pregnancy rate in female rabbits were recorded. However, according to the current state of knowledge the observed effects in animals do not seem to have relevance for clinical use.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Citric acid monohydrate (for pH adjustment)
Glycerol Egg lecithin Sodium oleate
Sodium hydroxide (for pH adjustment) all-rac-alpha-tocopherol Water for injections
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products for which compatibility has not been documented. See section 6.6.
Omeflex plus should not be given simultaneously with blood, see sections 4.4 and 4.5.
6.3 Shelf life
Unopened
2 years
After removing the protective overwrap and after mixing of the contents of the bag
After mixing the contents of the chambers the final emulsion is to be used immediately.
After admixture of compatible additives
From a microbiological point of view, the product should be used immediately after admixture of additives. If not used immediately after admixture of additives, in-use storage times and conditions prior to use are the responsibility of the user.
After first opening (spiking of the infusion port)
The emulsion is to be used immediately after opening of the container.
6.4 Special precautions for storage
Do not store above 25 °C.
Do not freeze. If accidentally frozen, discard the bag.
Keep the bag in the protective overwrap in order to protect from light.
6.5 Nature and contents of container
Omeflex plus is supplied in flexible multichamber bags of multilayer foil. The twin base port is made of polypropylene and styrene ethylene butylene styrene. The inner layer in contact with the solution consists of polypropylene. The multichamber bags contain:
- 1250 ml (500 ml of amino acids solution + 250 ml of fat emulsion + 500 ml of glucose solution)
- 1875 ml (750 ml of amino acids solution + 375 ml of fat emulsion + 750 ml of glucose solution)
- 2500 ml (1000 ml of amino acids solution + 500 ml of fat emulsion + 1000 ml of glucose solution).
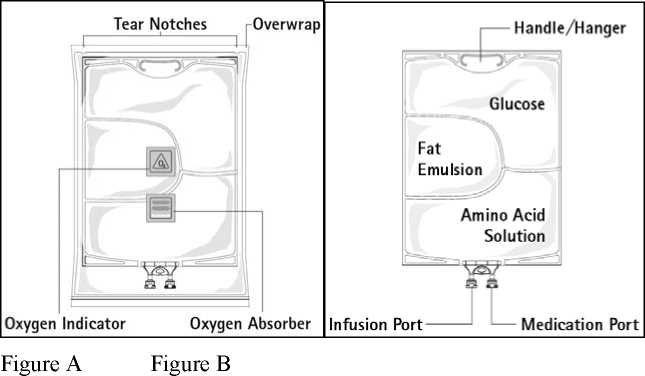
Figure A: The multichamber bag is packed in a protective overwrap. An oxygen absorber and an oxygen indicator are placed between the bag and the overwrap; the oxygen absorber sachet is made of inert material and contains iron hydroxide.
Figure B: The top chamber contains a glucose solution, the middle chamber contains a fat emulsion, and the bottom chamber contains an amino acid solution.
The top chamber and the middle chamber can be connected with the bottom chamber by opening the intermediate seams (peel seams).
The design of the bag permits mixing of the amino acids, glucose, lipids and electrolytes in a single chamber. Opening the peel seams results in sterile mixing to form an emulsion.
The different container sizes are presented in cartons containing five bags. Pack sizes: 5 x 1250 ml, 5 x 1875 ml and 5 x 2500 ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements for disposal.
Parenteral nutrition products should be visually inspected for damage, discolouration and emulsion instability before use.
Do not use bags which are damaged; neither overwrap nor the primary bag should be damaged. Use only if the peel seams between the chambers are intact, if amino acid and glucose solutions are clear and colourless up to straw-coloured, and the emulsion is a homogenous liquid with milky white appearance. Do not use if the solutions are discoloured or contain particulate matter. Do not use if the emulsion shows signs of phase separation (oil drops, oil layer).
Before opening the overwrap, check the colour of the oxygen indicator (see Figure A). Do not use if the oxygen indicator is pink. Use only if the oxygen indicator is yellow.
Preparation of the mixed emulsion
Strict adherence to aseptic handling principles must be complied with.
To open: Tear overwrap starting from the tear notches (Fig. 1). Remove the bag from its protective overwrap. Discard overwrap, oxygen indicator and oxygen absorber.
Visually inspect the primary bag for leaks. Leaky bags must be discarded, since the sterility cannot be guaranteed.
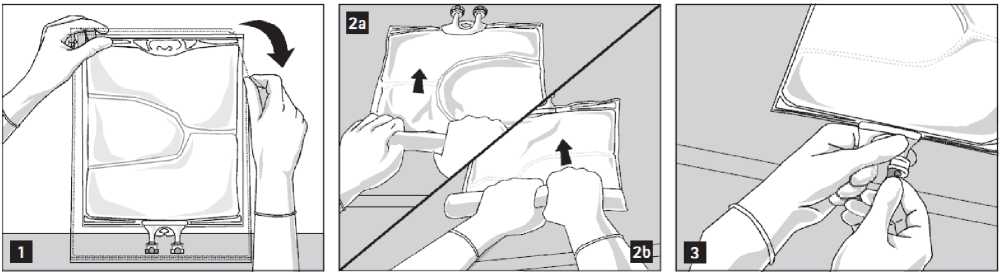
To open and mix the chambers sequentially, roll the bag with both hands, starting first by opening the peel seam that separates the top chamber (glucose) and the bottom chamber (amino acids) (Fig. 2a). Then continue applying pressure so that the peel seam separating the middle chamber (lipids) and the bottom chamber opens (Fig. 2b).
Addition of additives
After removing the aluminium seal (Fig. 3) one can add compatible additives via the medication port (Fig. 4).
Omeflex plus can be mixed with following additives:
N(2)-L-alanyl-L-glutamine
Aqua ad injectabilia
Calcium gluconate 10%
Sodium chloride 20%
Potassium chloride 14.9% Magnesium sulphate 50%
Potassium dihydrogenphosphate 1M Tracutil (trace elements)
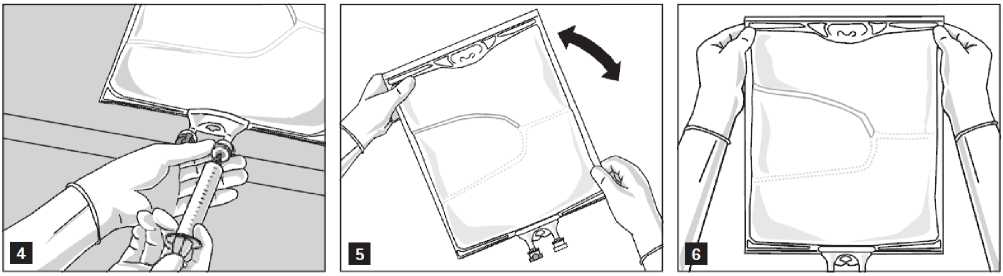
Mix the contents of the bag thoroughly (Fig. 5) and visually inspect the mixture (Fig. 6). There should be no signs of emulsion phase separation.
The mixture is a milky white homogenous oil-in-water emulsion.
Preparation _for infusion
The emulsion should always be brought to room temperature prior to infusion.
Remove the aluminium foil from the infusion port (Fig. 7) and attach the infusion set (Fig. 8). Use a non-vented infusion set or close the air vent when using a vented set. Hang the bag on an infusion stand (Fig. 9) and carry out infusion using the standard technique.
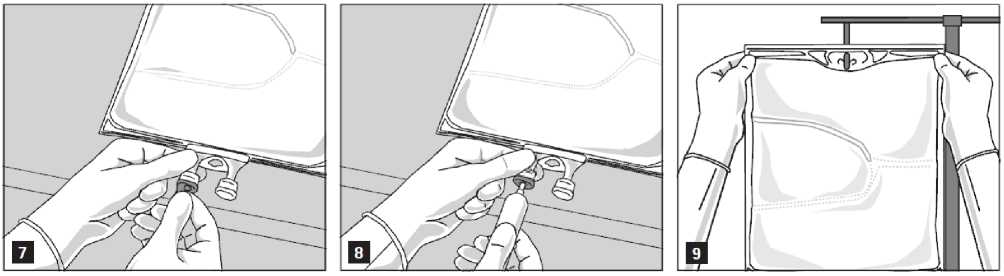
For single use only. Container and unused residues must be discarded after use.
Do not reconnect partially used containers.
If filters are used they must be lipid-permeable (pore size > 1.2 pm).
7 MARKETING AUTHORISATION HOLDER
B. Braun Melsungen AG Carl-Braun-StraBe 1 34212 Melsungen Germany
Postal address B. Braun Melsungen AG 34209 Melsungen Germany
Phone: +49-5661-71-0 Fax: +49-5661-71-4567
8 MARKETING AUTHORISATION NUMBER(S)
PL 03551/0143
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
12/07/2016
10 DATE OF REVISION OF THE TEXT
12/07/2016