Ondansetron 8 Mg Orodispersible Tablets
PACKAGE LEAFLET: INFORMATION FOR THE USER
MACEUTICALS
4 mg orodispersible tablets 8 mg orodispersible tablets
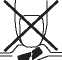
Fig. A
Fig. 1
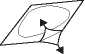
Fig. 2
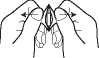
Fig. 3

Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet See section 4.
What is in this leaflet:
1. What Ondansetron is and what it is used for
2. What you need know before you take Ondansetron
3. How to take Ondansetron
4. Possible side effects
5. How to store Ondansetron
6. Contents of the pack and other information
1. What Ondansetron is and what it is used for
This medicine contains Ondansetron, which belongs to a group of medicines called anti-emetics which help to stop you feeling or being sick.
Ondansetron is used to treat nausea (feeling sick) and vomiting (being sick) caused by some medical treatments, such as chemotherapy or radiotherapy for cancer (in adults and children). It is also used to prevent nausea and vomiting in patients following an operation (adults only).
2. What you need to know before you take Ondansetron
Do not take Ondansetron
• if you are taking apomorphine (used to treat Parkinson's disease)
• if you are allergic to Ondansetron or any of the other ingredients of this medicine (listed in section 6),
• if you have ever had any allergic (hypersensitivity) reaction to other anti-emetics (for example granisetron or dolasetron).
Warnings and precautions
Talk to your doctor or pharmacist before taking
Ondansetron.
• if you have a blockage in your gut or suffer from severe constipation
• if you are due to have surgery to the adenoids or tonsils
• if you have a heart problem
• if you have liver problems
Other medicines and Ondansetron
Ondansetron may have an effect on other drugs and other drugs may have an effect on Ondansetron.
Tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines including medicines obtained without a prescription.
In particular it is important to tell your doctor if you are taking any of the following medicines:
• Apomorphine (a medicine used to treat Parkinson's disease), because severe drop in blood pressure and loss of consciousness were reported when Ondansetron was used with apomorphine.
• Medicines used to treat epilepsy (phenytoin, carbamazepine).
• Rifampicin (an antibiotic).
• Tramadol (a medicine used to treat pain).
• Medicines used to treat heart problems such as abnormal heart beats (anti-arrhythmics) and/or high blood pressure (beta-blockers).
• Medicines used to treat cancer.
Pregnancy
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
The safety of using Ondansetron during pregnancy has not been established. Therefore the use of Ondansetron is not recommended during pregnancy.
Breast-feeding
You should not breast-feed your infant whilst taking Ondansetron. Ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Ondansetron is unlikely to affect your ability to drive or operate machinery.
Ondansetron contains lactose, aspartame and ethanol
These tablets contain lactose (a type of sugar). If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
This medicine also contains aspartame which is a source of phenylalanine. This may be harmful for people with a condition called phenylketonuria.
This medicinal product contains a small amount of ethanol, less than 100 mg per tablet. May rarely cause severe hypersensitivity reactions and bronchospasm.
3. How to take Ondansetron
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Ondansetron orodispersible tablets should be taken by mouth. The tablet(s) will break up rapidly in the mouth and can then be swallowed. You should wash it down with a glass of water.
Important: Do not remove Ondansetron orodispersible tablets from their blister or pierce the foil until you are ready to take them.
Follow these instructions carefully:
The tablet(s) must be taken as follows:
In order to stop the tablet from breaking, do not push the tablet out of its blister.
Tear along the perforations of the foil to separate off one blister unit.
Remove the covering foil carefully. Start with the corner that is marked with an arrow.
Place the tablet on top of your tongue with dry hands. The tablet will dissolve very quickly, and you should then swallow it with water.
Fig. 4
Treatment of sickness (nausea and vomiting) in patients receiving chemotherapy or radiotherapy:
Adults (including the elderly): The recommended dose is 8 mg of Ondansetron 1 to 2 hours before chemotherapy or radiotherapy, followed by 8 mg of Ondansetron 12 hours later. To protect against delayed, or further, sickness a dose of 8 mg of Ondansetron may be continued twice a day for up to 5 days after treatment.
Infants and Children (aged 6 months and over) and adolescents (under 18 years old):
Your doctor will decide what dose of Ondansetron should be given. This will depend on the size and weight of the child.
Patients with liver problems:
The total daily dose should not be more than 8 mg.
To prevent sickness (nausea and vomiting) after an operation:
Adults: The usual dose is 16 mg before your operation or an 8 mg tablet one hour before the operation, then
- another 8 mg tablet eight hours after the first dose, then
- a further 8 mg tablet eight hours after the second dose
Ondansetron orodispersible tablets should start to work within one to two hours of taking a dose.
If you are sick (vomit) within one hour of taking an 8 mg dose:
- Take another 8 mg tablet
- Otherwise, do not take more Ondansetron orodispersible tablets than the label says.
If you take more Ondansetron than you should
If you have taken too many tablets it is important to contact your doctor as soon as possible or go to your nearest hospital casualty department immediately. Take the medicine packet with you, even if there are no tablets left, so that the doctor knows which tablets were taken.
If an overdose has been taken, symptoms may include problems with vision, low blood pressure (which could cause dizziness or faintness) or irregular heartbeat.
If you forget to take Ondansetron
If you forget to take a dose, take it as soon as you remember unless it is nearly time for your next dose. Do not take a double dose to make up for a forgotten dose.
If you stop taking Ondansetron
Do not stop taking your tablets, even if you are feeling well, without consulting a doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following effects happen, stop taking your tablets and tell your doctor immediately:
• An allergic reaction. The symptoms may include:
- Sudden wheezing and chest pain or tightness.
- Swelling of the eyelids, face, lips, mouth, tongue or throat.
- Difficulty breathing.
- Collapse.
- Skin rash.
• Fits (seizures).
• Chest pain.
• Temporary loss of vision which usually comes back within 20 minutes.
Side effects of Ondansetron
If you are concerned about any side effects please talk to your doctor. If any of the following side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Very common : may affect more than 1 in 10 people
• Headache.
Common: may affect up to 1 in 10 people
• Sensation of flushing and warmth.
• Constipation.
Uncommon: may affect up to 1 in 100 people
• Uncontrollable movements of the body, including upward rolling movement of the eyeballs.
• Fits (seizures). If this should occur seek urgent medical attention
• Irregular heartbeat, fast or slow heart rate.
• Chest pain. If this should occur seek urgent medical attention.
• Low blood pressure.
• Hiccups.
• An increase in liver function test results (most often in patients receiving chemotherapy with cisplatin).
Rare: may affect up to 1 in 1,000 people
• An immediate allergic reaction, which may be serious and include symptoms such as sudden wheezing and chest pain or tightness, swelling of the eyelids, face, lips, mouth, tongue or throat, difficulty breathing, collapse, skin rash. If this should occur, seek urgent medical attention.
• Visual disturbances e.g. blurred vision (though this has almost always been associated with an Ondansetron injection rather than tablets).
Very rare: may affect up to 1 in 10,000 people
• Temporary loss of vision which usually comes back within 20 minutes. If this should occur seek urgent medical attention. Temporary loss of vision has almost always been reported with Ondansetron injection, rather than tablets, and usually when given with chemotherapy containing cisplatin.
• Effects on heart rhythms that can be seen on an ECG (an electrical trace of your heart). Symptoms may include feeling light headed or loss of consciousness.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Ondansetron
Keep this medicine out of the sight and reach of children.
Store in the original package in order to protect from light.
Do not use this medicine after the expiry date, which is stated on the blister and carton after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via waste water or house hold waste. Ask your pharmacist how to throw away medicines you no longer require. These measures will help to protect the environment.
6. Contents of the pack and other information
What Ondansetron contains
- The active substance is ondansetron.
Each 4 mg tablet contains 4 mg of ondansetron. Each 8 mg tablet contains 8 mg of ondansetron.
- The other ingredients are mannitol (E421), crospovidone (TypeA), lactose monohydrate, microcrystalline cellulose, aspartame (E951), colloidal anhydrous silica, magnesium stearate, strawberry guarana flavour (maltodextrin, propylene glycol, artificial flavours containing amongst other ingredients [Benzyl alcohol, ethanol, potassium, propylene glycol, sodium, sulphites] and acetic acid).
What Ondansetron looks like and contents of the pack
Orodispersible tablet.
The 4 mg tablets are white to off-white, round tablets indented with '5' on one side and 'E' on the other side with a raised circular edge.
The 8 mg tablets are white to off-white, round tablets indented with '7' on one side and 'E' on the other side with a raised circular edge.
Ondansetron orodispersible tablets are available in packs of 6, 10, 30, 50 & 100 tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder:
Amneal Pharma Europe Limited 70 Sir John Rogerson's Quay Dublin 2 Ireland
Manufacturer:
APL Swift Services (Malta) Limited HF26, Hal Far Industrial Estate,
Hal Far, Birzebbugia, BBG 3000 Malta