Opticrom Hayfever 2% W/V Eye Drops
Is this leaflet hard to see or read? Phone 0845 372 7101 for help
PACKAGE LEAFLET:
INFORMATION FOR THE USER
Opticrom Hayfever 2% w/v Eye Drops sodium cromoglicate
SANOFlO
Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to use Opticrom Hayfever carefully to get the best results from it.
• Keep this leaflet. You may need to read it again
• Ask your pharmacist if you need more information or advice
• You must contact a doctor if your symptoms worsen or do not improve after 14 days
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist
In this leaflet:
1. What Opticrom Hayfever is and what it is used for
2. Before you use Opticrom Hayfever
3. How to use Opticrom Hayfever
4. Possible side effects
5. How to store Opticrom Hayfever
6. Further information
1. What Opticrom Hayfever is and what it is used for
Opticrom Hayfever 2% w/v Eye Drops (called Opticrom Hayfever in this leaflet) contain a medicine called sodium cromoglicate. This belongs to a group of medicines called anti-allergics.
It works by stopping the release of the natural substances in your eyes that can lead to an allergic reaction. Signs of an allergic reaction include itchy, watery, red or inflamed eyes and puffy eyelids.
These symptoms occur usually in the late spring or early summer when pollen from grasses or trees comes into contact with the eyes of people who are sensitive to them. This condition is sometimes called ‘hayfever eyes’ or by its medical term: seasonal allergic conjunctivitis.
2. Before you use Opticrom Hayfever
3 Do not use this medicine:
x If you or your child are allergic (hypersensitive) to sodium cromoglicate, or any of the other ingredients of Opticrom Hayfever (listed in Section 6: Further information)
Signs of an allergic reaction include: a rash, swallowing or breathing problems, swelling of your lips, face, throat, tongue and worsening of redness, itching or swelling of the eye or eyelid x If your child is under 6 years of age
x For other allergic eye symptoms (not caused by hayfever). Other things in everyday life can start allergies causing eye symptoms in certain people. For example contact with animals including pets, house dust mites, other particles and chemicals. Talk to your pharmacist or doctor.
Do not use this medicine if the above applies to you. If you are not sure, talk to your doctor or pharmacist before using Opticrom Hayfever.
1 Take special care with Opticrom Hayfever:
▲ You must be confident that you or your child’s irritated eyes are caused by hayfever, or have been previously diagnosed by your doctor to suffer from hayfever: You can be sure allergic eyes are caused by hayfever if:
• Both eyes are affected
• You also have a runny or blocked nose
• Your eyesight is not affected
• You know you suffer from hayfever and your symptoms coincide with the times of year when grass or tree pollen is high.
But if you or your child have:
• No nose symptoms
• Only one eye is affected
• Your sight is affected
Or if the eye symptoms:
• occur at the wrong time of year for hayfever
• are likely to be caused by another allergy, e.g. a pet or dust mite allergy
then you cannot be sure that the condition is due to hayfever and you should see your doctor or pharmacist before you use these drops.
If you are not sure if the above applies to you, talk to your doctor or pharmacist before using Opticrom Hayfever.
Pregnancy and breast-feeding
Talk to your doctor before using this medicine if you are pregnant, might become pregnant or think you may be pregnant.
If you are breast-feeding or planning to breast-feed, talk to your doctor or pharmacist before taking or using any medicine.
E9 Driving and using machines
You may have blurred eyesight straight after using this medicine.
If this happens, do not drive or use any tools or machines until you can see clearly.
Important information about some of the ingredients of Opticrom Hayfever
Opticrom Hayfever contains benzalkonium chloride. This may cause your eyes to become irritated.
Benzalkonium chloride is known to discolour soft contact lenses. Avoid contact with soft contact lenses.
For all other types of contact lenses:
Remove contact lenses prior to application and wait at least 15 minutes before reinsertion.

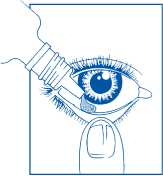

3. How to use Opticrom Hayfever
How to use this medicine
Adults (including the elderly) and children over 6 years
• Wash your hands
• Remove the cap from the bottle
• Tilt your head back
• Squeeze one or two drops inside the lower lid without touching your eye
• Close your eye
• Wipe away any excess liquid from the eyes with a clean tissue
• Always put the cap back on the bottle as soon as you have used it
• Repeat in the other eye How much to use
• One or two drops in each eye four times a day If you have any questions concerning the use of these drops in a child, or are unsure if the child has allergic eyes, you should seek advice from your doctor or pharmacist.
If your eyes are no better after 2 days of using the eye drops, see your doctor or pharmacist.
It is best to use your eye drops regularly every day while high pollen levels
are expected, to keep your eye condition controlled. If you find you need
to use them continuously for more than 14 days, then check with
your pharmacist or doctor before continuing
If you use more of this medicine than you should
Contact your doctor if you use the eye drops more frequently than the
recommended dose.
If you forget to use Opticrom Hayfever
If you forget a dose, use your drops as soon as you remember.
However, if it is nearly time for your next dose, skip the missed dose. Do not use a double dose to make up for a forgotten dose.
0If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Opticrom Hayfever can cause side effects, although not
everybody gets them.
Stop using Opticrom Hayfever and see a doctor as soon as possible if:
• The itching, redness or swelling gets worse. You may be allergic to these drops.
Talk to your doctor or pharmacist if any of the side effects gets serious or lasts longer than a few days, or if you notice any side effects not listed in this leaflet:
• Stinging or burning in your eyes or blurring of eyesight. This should only last for a short time and occurs immediately after using the eye drops
• Mild eye irritation
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www. m h ra.gov. u k/ye 11 owca rd
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Opticrom Hayfever
Keep this medicine in a safe place where children cannot see or reach it. Do not use Opticrom Hayfever after the expiry date which is stated on the label and carton. The expiry date refers to the last day of that month. Store below 30°C. Keep the bottle in the outer carton in order to protect from light.
Opticrom Hayfever is sterile when you buy it, so you must not keep it for more than four weeks after opening the bottle.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What Opticrom Hayfever contains
• The solution contains 2.0% w/v of the active substance, sodium cromoglicate
• The other ingredients are disodium edetate, benzalkonium chloride and purified water
What Opticrom Hayfever looks like and contents of the pack
Opticrom Hayfever is a clear colourless to pale yellow solution supplied in a 5 ml or 10 ml plastic dropper bottle with a tamperproof cap. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder Sanofi,
One Onslow Street, Guildford, Surrey, GU1 4YS, UK Tel: 0845 372 7101
email: uk-medicalinformation@sanofi.com Manufacturer
Sanofi Winthrop Industrie, Boulevard Industriel, 76580 Le Trait, France This leaflet does not contain all the information about your medicine. If you have any questions or are not sure about anything, ask your doctor or pharmacist.
This leaflet was last revised in 11/2013
© Sanofi, 2001 - 2013
