Optrex Infected Eye Drops

INFECTED EYE DROPS
iPlease read all of this leaflet carefully before you start [using your eye drops. Keep this leaflet. You may need to 'read it again.
'Remember - this medicine is for you. Never give it to [other people - it may harm them, even if their 'symptoms appear to be the same.
[1. What is your medicine for?
[Optrex Infected Eye Drops contain an antibiotic called 'chloramphenicol. It is used to treat bacterial infections [that affect the front surfaces of the eye.
[The most common type of infection in this area is called [acute bacterial conjunctivitis. When you have this condition, the white part of one or both of your eyes will [be red and/or your eyelids will be red or swollen. There will be a sticky discharge, which can make the eye [difficult to open in the morning and the eye may feel [‘gritty’or‘irritated’.
[Chloramphenicol eye drops are not suitable for treating >eye infections that have spread to the deeper layers of [the eye coverings or into the fluid within the eyeball. 'Antibiotic tablets or injections are needed to treat these [deeper, more serious infections.
2. Before using your medicine
Optrex Infected Eye Drops are recommended for use in [children aged 2 years and over, in adults and the jelderly. A child below the age of 2 years with an eye [infection should see a doctor.
[Do not use this product if you '• are allergic to chloramphenicol or any of the [ingredients shown under section 6 ‘What is in the pack?’ [• have ever had problems with your blood Qn particular very low numbers of blood cells)
[during previous treatment with chloramphenicol '• have a family history of blood problems such as low [white blood cell, red blood cell, or platelet counts [Do not use this product and seek advice from your [doctor if
[• your eyesight is affected (loss of sight, reduced [vision, blurred vision or halos around lights)
'• you have pain within the eye [• your eye has suffered a blow or other injury [• your eye is inflamed and you have a rash on the [scalp or face
[• the pupil of your eye looks unusual or your eye looks cloudy
[• your eyes are sensitive to light
• you have (or think you have) a foreign body in your eye, which has not been removed
• you have recently had an eye infection
• you suffer from or have ever suffered from glaucoma or dry eye syndrome
• you wear contact lenses. If you wear contact lenses and your contact lens practitioner or doctor has advised you to use this product, do not wear your lenses during the course of treatment. Soft contact lenses should not be replaced for 24 hours after completing the treatment.
• you are using any other eye drops or eye ointment
• you have had eye surgery or laser treatment in the last 6 months.
Tell your pharmacist before using this product if you
• are taking any other medicines
• suffer from any other eye problems.
Taking other medicines
If you are taking bone marrow depressant drugs (drugs which decrease the activity of the bone marrow, causing low blood cell counts) such as azathioprine or are receiving chemotherapy, seek the advice of your pharmacist or your doctor before using this product. Pregnancy and Breastfeeding If you are pregnant, planning to become pregnant or breastfeeding tell your doctor or pharmacist before using this product.
Driving and operating machinery
After using the drops, you may temporarily get blurred
vision. Do not drive or operate machinery until your
vision is clear. If in doubt, talk to your pharmacist or
doctor.
Important information about some of the ingredients in Optrex Infected Eye Drops
Contains phenylmercuric nitrate which may cause allergic reactions.


Pharmacode reads this way
o
Q.
n>


[I How to use
Applying the drops
[To be used in the eye(s) only.
[1. Check the seal is not broken before first use.
'2. Wash and dry your hands.
[3. Remove the plastic cap from the eye drop tip.
4. Tilt your head back while seated or lie down on your [back.
[5. Gently pull the lower eyelid downwards and look up. 16. Place the tip of the bottle close to your eye. Squeeze [the bottle and let one drop fall into the space between [the lower eyelid and the eye. Take care that the tip of [the bottle does not touch your eye, the skin around [your eye or your fingers.
'7. Close your eye for a moment.
8. When both eyes are to be treated, repeat for the other eye
9. Replace the cap straight away.
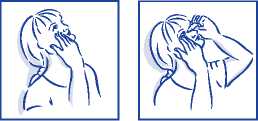
Whilst applying the drops, do not
• breathe on or touch the dropper nozzle
• touch the eyes or eyelids with the dropper nozzle
• share your eye drops with anyone else.
For external use only.
Dose
Apply one drop to the affected eye(s) every 2 hours for the first two days and 4 hourly thereafter. Use during waking hours only.
Length of treatment
The course of treatment is 5 days. Keep using these eye drops for 5 days. Do not stop just because you feel better - this could make your condition worse.
If your symptoms do not improve within 48 hours, consult your doctor. If your symptoms get worse, seek medical advice at once.
Do not use Optrex Infected Eye Drops for more than 5 days without consulting your doctor. Discard any remaining eye drops after the 5 day course of treatment.
If you foiget to use your drops
If you have only just missed a dose and it is a long time before the next dose is due, put in the missed dose of drops straight away. If you missed the dose some time ago and it is nearly time for your next dose, just put in the next dose of drops at the right time. Never double up on a dose to make up for the one you have missed.
4. Possible side effects
Some people may get one or more of these side effects. Some effects happen straight away but do not last long, others may only happen after several days of use.
• allergic reactions such as itching or rashes. If you get a severe reaction, with swelling or breathing problems get medical help straight away.
• changes in the blood (anaemia) leading to severe tiredness or easy bruising. If you experience these effects, stop using the drops immediately and tell your doctor.
• Discolouration or clouding of the eye surface
(cornea) or lens due to the preservative.
• The use of topical chloramphenicol may occasionally result in the overgrowth of other non susceptible organisms including fungi. If any new infection appears during treatment you should tell your doctor.
• blurred vision or mild burning or stinging when you put the drops in. These should subside quickly.
• irritation of the skin around the eyes, mercurialentis
(brown discolouration of the lens) and atypical band keratopathy (appearance of calcium on the cornea). These may be due to the preservative in the drops called phenyl mercuric nitrate.
If you do not feel well or are worried about your health, talk to a pharmacist or doctor.
If any of the side effects get serious or if you experience any side effect not listed in this leaflet please tell your doctor or pharmacist.
5. Storing your medicine
Replace the cap securely after use. Store at 2°C to 8°C. This is the temperature of most domestic refrigerators. Keep this and all medicines out of the sight and reach of! children. Do not use after the ‘use by’ date shown on the bottle. Discard any unused product within 28 days of opening. Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. What is in the pack?
Each pack contains 10ml of the drops. The drops contain 0.5% w/v chloramphenicol, which is equivalent to 5mg of chloramphenicol per millilitre. They also contain purified water, boric acid, borax and phenylmercuric nitrate.
Further information
If you have any questions or are not sure about anything, ask your pharmacist or doctor. They can obtain more information about this medicine if needed.
Licence holder: Optrex Limited, Slough, SL13UH, UK. Manufacturer: Reckitt Benckiser Healthcare International Ltd, Nottingham. NG90 2DB. PL00062/0051.
Leaflet revised May 2015.