Oroeze 0.15% W/V Oromucosal Spray

iceuticals Ltd
<ry
"0“
103448/LF/292/02
Benzydamine 0.15% w/v Oromucosal Spray
Benzydamine Hydrochloride
Read all of this leaflet carefully because it
contains important information for you.
This medicine is available without prescription.
However, you still need to use it carefully to
get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor or dentist if your symptoms worsen or do not improve after 7 days.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, dentist or pharmacist.
In this leaflet:
What Benzydamine Spray is and what it is used for
2. Before you use Benzydamine Spray
3. How to use Benzydamine Spray
4. Possible side effects
5. How to store Benzydamine Spray
6. Further information
1. What Benzydamine Spray is and what it is used for Benzydamine Spray is used for treating pain and inflammation (redness, swelling and a feeling of heat) in the throat or mouth. Benzydamine Spray contains benzydamine hydrochloride, one of a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs).
What this medicine does
Benzydamine Spray is used to treat many
painful conditions affecting the throat or mouth
including:
• Sore throat
• Sore tongue or gums
• Mouth ulcers
• Discomfort caused by dentures
• Pain after dental surgery
Benzydamine Spray works in the throat or mouth to help stop pain and inflammation (redness, swelling and a feeling of heat). It does this by stopping the formation of prostaglandins. Prostaglandins are natural substances made by the body when it is injured or has an infection. They cause an increase in the blood supply to the area of injury or infection. This results in pain, redness, swelling and a feeling of heat in that area. Stopping the formation of prostaglandins reduces these effects.
2. Before you use Benzydamine Spray
Do not use Benzydamine Spray
If you are allergic (hypersensitive) to benzydamine or any of the other ingredients of Benzydamine Spray (see section 6, ‘What Benzydamine Spray contains').
Take special care with Benzydamine Spray Do not use this spray near your eyes. If you gel it in your eyes accidentally, wash them immediately with cold water.
Using other medicines Tell your doctor, dentist or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding If you are pregnant, thinking of becoming pregnant or breast-feeding ask your doctor, dentist or pharmacist for advice before using Benzydamine Spray.
Important information about some of the non-active ingredients in Benzydamine Spray
Some of the non-active ingredients in your medicine may cause side effects:
• Methyl parahydroxybenzoate - this may cause allergic reactions (possibly delayed)
• Propylene glycol - this may cause skin irritatior
3. How to use Benzydamine Spray
Unless your doctor or dentist has advised otherwise, always use Benzydamine Spray as described in this leaflet. Check with your doctor, dentist or pharmacist if you are not sure. Benzydamine Spray is for use in the throat or mouth. It should be sprayed onto the sore area of the mouth or throat according to the following instructions:
Adults including the elderly
• Use 4 to 8 sprays every 1V2 to 3 hours. Children aged 6 to 12 years
• Use 4 sprays every 1V2 to 3 hours.
• Children over 12 should use the adult dose. Children under 6 years of age
• The dose is based on how much the child weighs.
• Do not use more than 4 sprays at any one time,
• Use one spray for each 4 kilograms that the child weighs but do not use more than 4 sprays at any one time.
• This dose should be used every 1V2 to 3 hours. How to use the spray
1. Always use the spray in the upright position.
2. Turn the spray arm, in either direction, until it is at right angles (90 degrees) to the body of the spray container as shown in picture 1. Do noi use the spray arm in any other position, as the medicine may not come out properly.
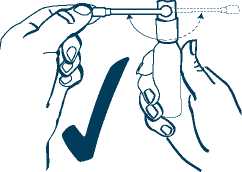
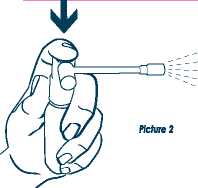
3. I he first time you use the spray, point the spray arm away from you and anyone else.
Then press the white plunger down firmly several times until a fine spray appears out of the end of the spray arm as shown in picture 2.
Your spray is now ready to use.
4. Point the spray arm at the sore part of your throat or mouth and press the white plunger down firmly. One press releases one spray of medicine.
5. Apply the correct number of sprays according to the instructions above. Then wipe the end of the spray arm with a clean tissue. This helps to prevent your spray blocking.
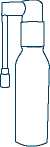
6. Turn the spray arm down into
the closed position as shown in picture 3. This stops any more sprays of your medicine
being released accidentally.
7. If your spray blocks or stops
working properly, return it to your pharmacist. Do not push anything into the end of the spray arm to try to clear a blockage. Havre 3
If you use more Benzydamine Spray than you should If you use too many sprays of Benzydamine Spray, contact your doctor, dentist or pharmacist for advice.
If you forget to use Benzydamine Spray If you forget to use your spray, do not worry. Apply the correct number of sprays as soon as you remember and then carry on using the spray every 1 '/2 to 3 hours as before.
• Do not use double the amount of sprays to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor, dentist or pharmacist.
4. Possible side effects
Like all medicines, Benzydamine Spray can cause side effects, although not everybody gets them. Side effects are generally minor.
If you have any of the following side effects while taking your medicine tell your doctor Immediately or go to hospital straight away: Severe allergic reaction which may include a red and lumpy skin rash, difficulty breathing, swelling of face, mouth, lips or eyelids, unexplained high temperature (fever) and feeling faint. If the swelling affects your throat and makes breathing and swallowing difficult, go to hospital straight away.
Itchy rash, sometimes with pale, raised areas of skin with red edges (urticaria).
Your skin becoming more sensitive to sunlight than normal causing an itchy, red, scaly rash, sometimes with blisters.
Tightening of the throat or chest making it difficult to breathe.
Uncommon (may affect up to 1 in 100 people):
A feeling of numbness in your mouth A stinging feeling in your mouth:
- this stinging usually disappears as you continue to use the spray
- if the stinging does not go away stop using the _spray and talk to your doctor, dentist or pharmacist
See section 2 (Important information about some of the non-active ingredients in Benzydamine Spray) for side effects that may be caused by some of the non-active ingredients in your medicine. Reporting of side effects
If you get any side effects, talk to your doctor, dentist or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Benzydamine Spray
Keep out of the reach and sight of children.
Do not use after the expiry date stated on the carton and the base of the can. The expiry date refers to the last day of that month.
Do not store above 25°C. Keep the spray in the outer carton.
Once you have started using the spray, do nol keep it for more than 6 months.
Do not use if you notice any damage to the spray container or spray arm. Return it to your pharmacist. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What Benzydamine Spray contains
• The active substance is benzydamine hydrochloride. Each spray from the spray arm contains 262.5 micrograms of benzydamine hydrochloride.
• The other ingredients are glycerol, polysorbate 20, saccharin sodium, ethanol 96%, methyl parahydroxybenzoate,aniseed flavour (contains ethanol 95%), peppermint flavour (contains propylene glycol), water. (See section 2 for important information about some of these non-active ingredients.)
What Benzydamine Spray looks like and contents of the pack Benzydamine Spray is an oromucosal spray (a spray for use in the throat or mouth). It is a clear solution with a smell of peppermint. It is contained in a spray container fitted with a metering pump (a pump that delivers a measured amount ol medicine) and nozzle (spray arm).
Each spray container holds 30ml of medicine. Marketing Authorisation Holder Focus Pharmaceuticals Limited, Capital House, 85 King William Street, London EC4N 7BL, UK.
Tel: 01283 495 280 Fax: 01283 495 290 Email: medinfo@focuspharma.co.uk Manufacturer PPF Hasco-Lek S.A, ul. Eugeniusza Kwiatkowskiego 9, 55-011 Siechnice, Poland.
For any information about this medicinal product, please contact the Marketing Authorisation Holder, details provided above. For information in large print, audio CD or Braille please telephone 01283 495 280 or email medinfo@focuspharma.co.uk.
This leaflet was last revised in March 2016.
|
/ |
\ FOCUS |
|
Version No.: |
103448/LF/292/02 |
|
Product Name: |
Benzydamine 0.15% |
|
w/v Mouth Spray | |
|
Pack Size: |
30 ml |
|
Component: |
Leaflet |
|
SKU: |
103448 |
|
Market: |
UK |
|
Production Site: |
Hasco-Lek S.A |
|
Revision No.: |
1 |
|
Revision Date: |
30/03/2016 |
|
Revised by: |
PAT |
|
CRF: |
AMCo.CRF.101.2016 |
|
V |
y |
|
r Dimension: |
160x240 mm |
\ |
|
Commodity No.: |
N/A | |
|
Pharma Code: |
N/A | |
|
Core Spec Ref: |
N/A | |
|
DCMF: |
N/A | |
|
Print Colours: |
302 C | |
|
Non-Print Colours: Cutter | ||
|
Tech App. Date: |
08/04/2016 | |
|
Min. Font Size: |
7.5 pt | |
|
V |
y | |
/-N
REGULATORY AUTHORITY APPROVAL CONFIRMATION
Confirmation that this artwork has been approved by the appropriate market authority (if applicable, e.g. MHRA, HPRA, etc.) and that Amdipharm have license approval to distribute this component for sale in the relevant market.
Signature ...................................................................
Name .........................................................................
Date ...........................................................................
V_y
PAGE 1 OF 1
v_y