Otomize
Out of date information, search another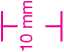
Pharmacode: 10mm high. First Bar/Left hand bar starts 10mm down.
40 mm -1
-120 mm

dexamethasone, neomycin sulphate, acetic acid




OTOMIZE
Please read right through this leaflet before you start using this medidne. This medicine is available on prescription only.
• Keep this leaflet you may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
In this leaflet:
1. What Otomize does
2. Check before you use Otomize
3. How to use Otomize
4. Possible side effects
5. How to store Otomize
6. Further information
1. What Otomize does
Otomize is used to treat infection and inflammation of the outer ear.
Otomize contains three active ingredients. Dexamethasone is a topical corticosteroid medicine which is used to control inflammation. Neomycin sulphate is a broad spectrum antibiotic to fight infections and acetic acid is an antibacterial agent that also helps to clear infections.
2. Check before you use Otomize
Do not use Otomize:
• if you have ever had an allergic reaction to
dexamethasone, neomycin sulphate, acetic acid or to any of the other ingredients (listed in Section 6).
• anywhere other than in the ear. Do not inhale in the mouth or nose or spray near the eyes.
• if you have a perforated ear drum or have a grommet fitted in the affected ear.
• in children under 2 years of age
Take special care with Otomize
• Excessive use (high doses or prolonged use) of this product on open wounds or damaged skin may cause deafness. This is particularly important to consider when used on children.
• Do not swallow any of the spray. If swallowed (especially by a child) contact your doctor or casualty department immediately.
Pregnancy and breast feeding
Talk to your doctor before using Otomize if you
are pregnant, trying to become pregnant, or
breast feeding.
3. How to use Otomize
Before using a new bottle of Otomize:
• Shake well before use.
• Press the pump down several times until you get a fine spray.
• Make sure you do not point the nozzle at yourself or someone else.
• Each time the pump is pressed down one measured dose will be delivered.
• If you have not used Otomize for more than a week, press the pump a few times until you get a fine spray again, and then follow the instructions above.
OT/LFT/UK/1/B
Front
For external use only.

Using Otomize:
Adults and children over the age of 2 years.
• Shake well before use.
• Place the nozzle tip gently into your ear.
• Press the pump once to deliver the correct dose.
• Spray one measured dose into each affected ear three times a day, or as directed by your doctor.
• If you forget to use Otomize do not worry.
Use it as soon as you remember then go on as before.
• The nozzle tip must always be in place when you use the spray. You can remove it for cleaning but always click it back into place afterwards.
H* Continue using Otomize until two days after symptoms have gone.
• Stop using the product and consult a doctor if your condition worsens or does not improve after 7 days treatment. • Do not use more than the recommended dose.
4. Possible side effects
Like all medicines, Otomize can have side effects, but not everyone gets them.
Stop using the medicine and tell your doctor if you experience:
• Sensitivity or allergic reactions (irritation or a rash).
The following side effects may also occur, but usually go away after the first few days of treatment:
• Temporary stinging or a burning sensation.
5. How to store Otomize
Keep out of the reach and sight of children.
Do not use this medicine after the 'EXP' date shown on the pack.
Do not store above 25°C.
Do not freeze.
Keep container upright in the outer carton.
Use within one month of first use.
6. Further information
Active ingredients Dexamethasone 0.1 % w/w, Neomycin Sulphate 0.5% w/w (3250 lU/ml), Acetic Acid (glacial) 2.0% w/w.
Other ingredients Macrogol (2) stearyl ether, macrogol (20) stearyl ether, stearyl alcohol, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216) and purified water.
Otomize is supplied in packs containing 5ml as an aqueous suspension. The spray is non-pressurised and does not contain CFCs.
The marketing authorisation holder is
Forest Laboratories UK Ltd, Dartford, Kent, DA2 6SL, UK and all enquiries should be sent to this address.
The manufacturer is Puma Pharmaceuticals NV, Rijksweg 17, 2870 Puurs, Belgium.
PL 00108/0332
Legal category: Prescription Only Medicine.
This leaflet was last revised in November 2011.
Otomize is a registered trade mark of Forest Laboratories UK Ltd.
If you do get any side effects, even those not mentioned in this leaflet, tell your doctor or pharmacist.
PH! Forest
OT/LFT/UK/1/B
Back