Otrivine Congestion Relief 0.1% Nasal Spray
ll) NOVARTIS
E
9 mm

T
(D
"O
o
o
03
J=
Q_

72 mm
EXT
E
Otrivine’
Congestion
Relief
0. 1. Nasal Spray
Xylometazoline Hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This medicine is available without a prescription. However, you still need to use Otrivine Congestion Relief 0.1% Nasal Spray carefully to get the best results from it.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- You must contact a doctor if the symptoms worsen or do not improve after 7 days.
- If any of the side effects gets serious, or if you notice any side effect not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Otrivine Congestion Relief 0.1% Nasal Spray is and what it is used for
2. Before you use Otrivine Congestion Relief 0.1% Nasal Spray
3. How to use Otrivine Congestion Relief 0.1% Nasal Spray
4. Possible side effects
5. How to store Otrivine Congestion Relief 0.1% Nasal Spray
6. Further information
1. What Otrivine Congestion Relief 0.1% Nasal Spray is and what it is used for
Otrivine Congestion Relief 0.1% Nasal Spray is for application in the nose to give relief from:
• nasal congestion (blocked nose, including colds)
• perennial and allergic rhinitis (recurring inflammation of the nasal mucous membranes, including hay fever)
• sinusitis.
Otrivine Congestion Relief 0.1% Nasal Spray contains the active ingredient xylometazoline hydrochloride which helps to open up and clear the nasal passages by reducing the excessive nasal secretions and returning the swollen blood vessels to their normal size.
2. Before you use Otrivine Congestion Relief 0.1% Nasal Spray
DO NOT use the spray if you:
• are allergic to xylometazoline hydrochloride or any of the other ingredients in the spray (see Section 6)
• have had recent neurosurgery
• have narrow angle glaucoma.
Do not use this spray for children under 12 years old.
Take special care
Before using the spray tell your doctor if you:
• have high blood pressure, heart or circulatory disease
• have an overactive thyroid
• have diabetes mellitus
• are pregnant or breast-feeding.
Do not to take decongestants for more than 7 days in a row. Prolonged or excessive use can cause the stuffiness in the nose to return or worsen. Keep away from the eyes and mouth. Like other products for the relief of blocked nose, this product can give rise to sleep disturbances, dizziness and tremor in very sensitive individuals. Consult your doctor if these symptoms have proven troublesome to you.
Using other medicines
You should not use Otrivine Congestion Relief 0.1% Nasal Spray if you are taking certain medicines used for treatment of depression (monoamine oxidase inhibitor (MAOI) or tri-cyclic and tetra-cyclic antidepressants).
Please tell your doctor or pharmacist if you are taking or have recently taken any medicines including those obtained without a prescription.
Pregnancy or breast-feeding
Do not use Otrivine Congestion Relief 0.1% Nasal Spray during pregnancy.
If you are breast-feeding, Otrivine Congestion Relief 0.1% Nasal Spray should only be used if recommended by your doctor.
Driving and using machinery
Otrivine has very little or no effect on your ability to drive or use machines. If you are experiencing blurred vision then do not drive or operate machines.
Important information about some of the other ingredients:
The medicine contains polyoxylhydrogenated castor oil (2.750 mg/ml) which may cause skin reactions.
3. How to use Otrivine Congestion Relief 0.1% Nasal Spray
For Adults and Children over 12 years of age: one spray in each nostril 1 to 3 times daily as needed.
E
E
o
CM
-»j
145 mm
ll) NOVARTIS
E
Administration
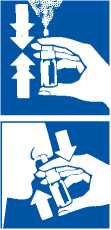
1. Blow the nose gently.
2. Remove protective cap.
3. Do not cut the nozzle.
The metered dose spray is ready to prime before use.
4. Before the first application, prime the pump by actuating (depressing the pump) 4 times. Once primed the pump will normally remain charged throughout regular daily treatment periods. Should the spray not be ejected during the full actuation, the pump will need to be re-primed by actuating 4 times. Be very careful not to spray in the eyes or mouth.
5. Hold the bottle upright with thumb under base and nozzle between two fingers.
6. Lean forward slightly and insert the nozzle into the nostril.
7. Spray and breathe in gently through the nose at the same time.
8. Repeat with the other nostril.
9. Clean and dry the nozzle before replacing back the cap right after use.
To avoid possible spread of infection, this pack should only be used by one person.
Do not exceed the recommended dosage.
If you use more Otrivine Congestion Relief 0.1% Nasal Spray than you should, consult your doctor or pharmacist immediately.
If you forget to use Otrivine Congestion Relief 0.1% Nasal Spray
Do not take a double dose to make up for a forgotten one.

9 mm
Allergic reactions (skin rash, itching), blurred vision, irregular or fast heartbeat.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of children.
Do not use after the expiry date which is stated on the bottle and carton after ‘EXP'.
The expiry date refers to the last day of that month.
Do not store above 25 °C.
Store in the original package.
Do not use this bottle for more than 28 days after opening it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.
These measures will help to protect the environment.
Like all medicines, Otrivine Congestion Relief 0.1% Nasal Spray can cause side effects, although not everybody gets them. Local discomfort may be felt after applying the spray if you have sensitive nasal passages.
STOP using Otrivine Congestion Relief 0.1% Nasal Spray and seek medical help immediately
if you have any of the following which may be signs of an allergic reaction:
difficulty breathing or swallowing, swelling of the face, lips, tongue or throat, severe itching of the skin, with a red rash or raised bumps.
Common side effects (may affect between 1 and 10 in every 100 patients).
Dryness or irritation of the nasal mucosa, nausea, headache, local burning sensation.
Very rare side effects (may affect less than 1 in every 10,000 patients).
What Otrivine Congestion Relief 0.1% Nasal Spray contains
The active ingredient is Xylometazoline hydrochloride (0.1% w/v). Each ml contains 1 mg of xylometazoline hydrochloride.
The other ingredients are: Sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, sodium chloride, disodium edetate, levomenthol, cineole, sorbitol, polyoxylhydrogenated castor oil (macrogol glycerol hydroxystearate) and purified water.
What Otrivine Congestion Relief 0.1% Nasal Spray looks like and the contents of the pack Otrivine Congestion Relief 0.1% Nasal Spray is a nasal metered-dose spray containing a white solution with menthol and eucalyptol odour, in a bottle with a pump designed to deliver the correct amount of product.
The content of the pack is 10 ml.
Marketing Authorisation Holder
Novartis Consumer Health, Camberley, GU15 3YL, UK.
Manufacturer
Novartis Consumer Health GmbH, Zielstattstrasse 40, 81379, Munich, Germany.
This leaflet was last revised in April 2014.
Otrivine® is a registered trademark of Novartis AG.
GB 910883 - 148649 (!> NOVARTIS
5. How to store Otrivine Congestion Relief 0.1% Nasal Spray


E
E
o
04
K
->j
145 mm I