Ovranette 150/30 Micrograms Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Ovranette® 150/30 micrograms Coated Tablets.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains 150 micrograms levonorgestrel and 30 micrograms ethinylestradiol.
Excipients with known effect: Each tablet also contains 32.97 mg lactose monohydrate and 22.023 mg sucrose.
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM Coated tablet.
White, shiny, sugar coated-tablet with a smooth surface.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Oral contraception Treatment of endometriosis
Treatment of spasmodic dysmenorrhoea and premenstrual tension
Treatment of functional uterine bleeding (menorrhagia, metrorrhagia, metropathia
haemorrhatica)
Emergency treatment of acute uterine bleeding
4.2 Posology and method of administration
Posology
Adults
How to take Ovranette
Tablets must be taken orally in the order directed on the blister package at about the same time every day, with some liquid if necessary.
Regular daily intake of tablets for 21 consecutive days is important for the preservation of contraceptive efficacy.
One tablet is to be taken daily for 21 consecutive days. Each subsequent pack is started after a 7-day tablet-free interval during which time a withdrawal bleed occurs. This usually starts on day 2-3 after the last tablet and may not have finished before the next pack is started.
How to start Ovranette
No preceding hormonal contraceptive use in the past month
Tablet-taking should start on day 1 of the woman’s natural cycle (i.e. the first day of her menstrual bleeding). Additional contraception (barriers and spermicides) is not required.
Changing from another combined oral contraceptive (COC)
Changing from another 21 day combined oral contraceptive: The first tablet of Ovranette should be taken on the first day immediately after the end of the previous oral contraceptive course. Additional precautions are not required. A withdrawal bleed should not be expected until the end of the first pack.
Changing from an Every Day (ED) 28 day combined oral contraceptive: The first tablet of Ovranette should be taken on the day immediately after the day on which the last active pill in the ED pack has been taken. The remaining tablets in the ED pack should be discarded. Additional precautions are not required. A withdrawal bleed should not be expected until the end of the first pack.
Changing from a progestogen-only-pill (POP)
The first tablet of Ovranette should be taken on the first day of menstruation even if the POP for that day has already been taken. The remaining tablets in the POP pack should be discarded. Additional precautions are not required.
Post-partum and post-abortum use
After pregnancy, combined oral contraception can be started in non-lactating women 21 days after a vaginal delivery, provided that the patient is fully ambulant and there are no puerperal complications (see section 4.4). If the pill is started later than 21 days after delivery, then alternative contraception (barriers and spermicides) should be used until oral contraception is started and for the first 7 days of pill-taking. If unprotected intercourse has taken place after 21 days post partum, then oral contraception should not be started until the first menstrual bleed after childbirth.
The use of COCs is generally not recommended until the nursing mother has weaned her child (see section 4.6).
After miscarriage or abortion oral contraception may be started immediately.
Other indications
Endometriosis: Continuous treatment with two tablets daily.
Spasmodic dysmenorrhoea, premenstrual tension: Dosage as for oral contraception.
Functional uterine bleeding: Two tablets are taken daily on a cyclic basis as for oral contraception. In the first one or two cycles it may be necessary to give four tablets, or in exceptional cases, five.
Emergency treatment of acute uterine bleeding: Four tablets are given initially and, if necessary, 4-8 tablets daily.
Special circumstances requiring additional contraception
Management of Missed Tablets
Contraceptive reliability may be reduced if tablets are missed and particularly if the missed tablets extend the tablet-free interval. If tablets were missed in the first week of the cycle and intercourse took place in the week before the tablets were missed, the possibility of a pregnancy should be considered.
• Provided that the user is less than 12 hours late in taking any tablet, she should take it as soon as she remembers and further tablets should be taken at the usual time.
• If she is more than 12 hours late in taking any tablet, contraceptive protection may be reduced.
• The user should take the last missed tablet as soon as she remembers, even if this means taking two tablets in one day. She then continues to take tablets at her usual time. In addition, a back-up method such as the condom should be used for the next 7 days.
• If these 7 days run beyond the last tablet in the current pack, the next pack must be started as soon as the current pack is finished; no gap should be left between packs. This prevents an extended break in tablet taking which may increase the risk of escape ovulation. The user is unlikely to have a withdrawal bleed until the end of the second pack but she may experience spotting or breakthrough bleeding on tablet taking days.
• If the user does not have a withdrawal bleed at the end of the second pack, the possibility of pregnancy must be ruled out before resuming tablet taking from the next pack.
Gastro-intestinal Upset
If vomiting or severe diarrhoea occurs within 4 hours after tablet taking, tablet absorption may be incomplete. Repeat the missed dose as soon as possible. The general advice for women using COCs who have persistent vomiting or severe diarrhoea for more than 24 hours is to follow the instructions for missed pills.
How to delay a period
To delay a period the woman should continue with another pack of Ovranette without a tablet-free interval. The extension can be carried on for as long as wished until the end of the second pack. During the extension the woman may experience breakthrough-bleeding or spotting.
Regular intake of Ovranette is then resumed after the usual 7 day tablet-free interval.
Elderly
Not applicable.
Paediatric population Not applicable.
Method of administration
For oral administration.
4.3 Contraindications
Ovranette should not be used in the presence of any of the conditions listed below. Should any of the conditions appear for the first time during Ovranette use, the product should be stopped immediately.
• Breastfeeding: <6 weeks postpartum
• History of confirmed venous thromboembolism (VTE). Known risk factors for VTE.
• Arterial thrombotic disorders (Cerebrovascular accident or coronary artery disease) or a history of these conditions, or prodromal conditions (e.g. angina pectoris and transient ischaemic attack)
• Smoking in women aged >35 years who smoke >15 cigarettes per day
• Severe or uncontrolled hypertension
• Known thrombogenic mutations, thrombogenic valvulopathies or congenital heart disease
• History of migraine with focal neurological symptoms
• Diabetes mellitus with vascular involvement
• Systemic Lupus Erythematosus (SLE) with positive (or unknown) antiphospholipid antibodies
• The presence of a severe or multiple risk factor(s) for venous or arterial thrombosis may also constitute a contraindication (see section 4.4)
• Acute or severe chronic liver diseases, current or previous, as long as liver function values have not returned to normal
• Presence or history of liver tumours (benign or malignant)
• Known or suspected carcinoma of the breast or other known or suspected estrogen-dependent neoplasia
• Undiagnosed vaginal bleeding
• Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Assessment of women prior to starting oral contraceptives (and at regular intervals thereafter) should include a personal and family medical history of each woman. Physical examination should be guided by this and by the Contraindications (section 4.3) and Special warnings and precautions for use (section 4.4) for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, breast, abdominal and pelvic examination, including cervical cytology.
Before starting treatment, pregnancy must be excluded.
In cases of undiagnosed abnormal genital bleeding, adequate diagnostic measures are indicated.
Warnings
Patients should be counselled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
The suitability of a combined oral contraceptive should be judged according to the severity of such conditions in the individual case, and should be discussed with the patient before she decides to take it.
Minimising exposure to estrogens and progestogens is in keeping with good principles of therapeutics. For any particular estrogen/progestogen combination, the dosage regimen prescribed should be one that contains the least amount of estrogen and progestogen that is compatible with a low failure rate and the needs of the patient.
Conditions requiring supervision:
Ovranette can be used in women with a condition that falls into WHO category 3, but as the theoretical or proven risks usually outweigh the advantages of using the COC, the decision to prescribe the COC must be made using clinical judgment and in consultation with the woman. If any of these conditions appear for the first time during Ovranette use, consideration should be given to stopping COC use.
• Breastfeeding - >6 weeks to <6 months postpartum (primarily breastfeeding)
• Postpartum (in non-breastfeeding women) - <21 days
• Smoking - aged >35 years and smoking <15 cigarettes/day
• Cardiovascular Disease - Multiple risk factors for arterial cardiovascular disease (such as older age, smoking, diabetes and hypertension)
• Hypertension - adequately controlled hypertension: elevated blood pressure levels systolic 140-159 mmHg or diastolic 90-94 mmHg
• Known Hyperlipidaemias
• Migraine Headaches - past history of migraine with aura; new onset migraine (see below)
• Breast Cancer - past and no evidence of current disease for 5 years
• Diabetes - with mild vascular disease or mild nephropathy, retinopathy, or neuropathy
• Gallbladder Disease - current or medically treated symptomatic gallbladder disease
• History of Cholestasis - past COC-related
• Viral Hepatitis - acute or flare.
Reasons for stopping Ovranette immediately:
• Occurrence of migraine in patients who have never previously suffered from it. Exacerbation of pre-existing migraine. Any unusually frequent or unusually severe headaches.
• Any kind of acute disturbance of vision
• Suspicion of thrombosis or infarction including symptoms such as unusual pains in or swelling of the legs, stabbing pains on breathing, persistent cough or coughing blood, pain or tightness in the chest
• Significant rise in blood-pressure
• Jaundice
• Clear exacerbation of conditions known to be capable of deteriorating during oral contraception or pregnancy (see “Other” in section 4.4).
If oral contraception is stopped for any reason and pregnancy is not desired, it is recommended that alternative non-hormonal methods of contraception (such as barriers or spermicides) are used to ensure contraceptive protection is maintained.
Circulatory Disorders
Use of COCs is associated with an increased risk of venous and arterial thrombotic and thromboembolic events. The presence of one serious or multiple risk factors, depending on type and severity, for venous or arterial disease, may constitute an unacceptable level of risk (see section 4.3).
Venous Thrombosis and Thromboembolism
Use of COCs increases the risk of venous thrombotic and thromboembolic events. Reported events include deep venous thrombosis and pulmonary embolism.
The use of any COC carries an increased risk of venous thrombotic and thromboembolic events compared with no use. The excess risk is highest during the first year a woman ever uses a combined oral contraceptive. This increased risk is less than the risk of venous thrombotic and thromboembolic events associated with pregnancy which is estimated as 60 cases per 100,000 woman-years. Venous thromboembolism is fatal in 1-2% of cases.
Some epidemiological studies have reported a greater risk of VTE for women using combined oral contraceptives containing ethinylestradiol, mostly at a dose of 30pg, and desogestrel or gestodene (the so-called third generation pills) than for women using pills containing levonorgestrel (the so-called second generation pills).
The spontaneous incidence of VTE in healthy non-pregnant women (not taking any oral contraceptive) is about 5 cases per 100,000 women per year. The incidence in users of the second generation pills (such as Ovranette) is about 15 per 100,000 women per year of use. The incidence in users of third generation pills is about 25 cases per 100,000 women per year of use: this excess incidence has not been satisfactorily explained by bias or confounding. The level of all these risks of VTE increases with age and is likely to be further increased in women with other known risk factors for VTE. Caution must be exercised when prescribing COCs for such women.
The risk for venous thromboembolic complications in COCs users increases with:
• A personal or family history of venous thrombotic/thromboembolic events; certain inherited and acquired thrombophilias (see below)
• Obesity (body mass index of 30 kg/m2 or over)
• Recent delivery or second-trimester abortion
• Prolonged immobilisation, major surgery or trauma with increased risk of thrombosis (see below)
• Increasing age
• Systemic lupus erythematosus (SLE).
There is no consensus about the possible role of varicose veins and superficial thrombophlebitis in venous thromboembolism.
The relative risk of post-operative thromboembolic complications has been reported to be increased two- or four-fold with the use of COCs. If feasible, COCs should be discontinued for at least 6 weeks prior to and for two weeks after elective surgery with increased risk of thrombosis, or during prolonged immobilization.
Symptoms of venous thrombotic/thromboembolic events can include
• Severe pain in the calf of one leg; swelling of the lower leg
• Sudden breathlessness, chest pain
• Sudden partial or complete loss of vision.
Arterial Thrombosis and Thromboembolism
Epidemiological studies have suggested an association between the use of COCs and an increased risk for arterial thrombotic and thromboembolic events - primarily myocardial infarction and cerebrovascular events (transient ischaemic attack or stroke).
Cigarette smoking increases the risk of serious cardiovascular adverse reactions from COC use. This risk increases with age and with the extent of smoking, and is quite marked in women over 35 years of age. Women who use COCs should be strongly advised not to smoke.
The risk of arterial thrombotic and thromboembolic events is further increased in women with underlying risk factors (see below) and caution must be exercised when prescribing COCs for such women.
Examples of risk factors for arterial thrombotic and thromboembolic events include:
• Increasing age
• Smoking, especially over the age of 35
• Family history of arterial thromboembolic events; certain inherited and acquired thrombophilias (see below)
• Hypertension
• Dyslipoproteinaemias
• Thrombogenic valvular heart disease, atrial fibrillation
• Obesity (body mass index of 30 kg/m2)
• Diabetes
• Systemic Lupus Erythematosus (SLE)
• Migraine.
The onset or exacerbation of migraine or development of headache with a new pattern that is recurrent, persistent or severe requires discontinuation of COCs and evaluation of the cause. COC users with migraine (particularly migraine with aura) may be at increased risk of stroke (see section 4.3).
Symptoms of arterial thrombotic/thromboembolic events or of a cerebrovascular accident can include:
• Sudden severe pain in the chest, whether or not it radiates to the left arm
• Sudden onset of coughing; for no apparent reason
• Any unusual, severe, prolonged headache
• Sudden partial or complete loss of vision or diplopia
• Slurred speech or aphasia
• Vertigo
• Collapse with or without focal seizure
• Weakness or very marked numbness suddenly affecting one side or one part of the body, motor disturbances.
Other factors affecting circulatory events
Other medical conditions which have been associated with adverse vascular events include chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis) and sickle cell disease.
Biochemical factors that may be indicative of hereditary or acquired predisposition for venous or arterial thrombosis include Activated Protein C (APC) resistance, hyperhomocysteinemia, antithrombin-III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant).
Tumours
Numerous epidemiological studies have been reported on the risks of ovarian and endometrial cancer, in women using combined oral contraceptives. The evidence is clear that high dose combined oral contraceptives offer substantial protection against both ovarian and endometrial cancer. However, it is not clear whether low dose COCs confer protective effects to the same level.
Breast Cancer
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs). The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The additional breast cancers diagnosed in current users of COCs or in women who have used COCs in the last ten years are more likely to be localised to the breast than those in women who never used COCs.
Breast cancer is rare among women under 40 years of age whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer (see bar chart).
Estimated cumulative numbers of breast cancers per 10,000 women diagnosed in 5 years of use and up to 10 years after stopping COCs, compared with numbers of breast cancers diagnosed in 10,000 women who had never used COCs
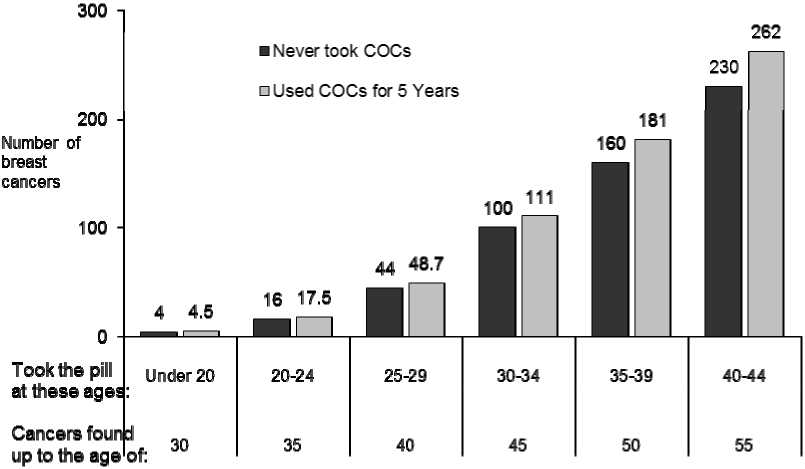
The most important risk factor for breast cancer in COC users is the age women discontinue the COC; the older the age at stopping, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the 10 years after stopping COC use such that by 10 years there appears to be no excess.
The possible increase in risk of breast cancer should be discussed with the user and weighed against the benefits of COCs taking into account the evidence that they offer substantial protection against the risk of developing certain other cancers (e.g. ovarian and endometrial cancer).
Cervical Cancer
The most important risk factor for cervical cancer is persistent HPV infection. Some epidemiological studies have indicated that long-term use of COCs may further contribute to this increased risk but there continues to be controversy about the extent to which this finding is attributable to confounding effects, e.g. cervical screening and sexual behaviour including use of barrier contraceptives.
Hepatic Neoplasia/Liver Disease
Benign and malignant liver tumours have been reported very rarely in users of COCs. The risk appears to increase with duration of COC use. In isolated cases these tumours have lead to life threatening intra-abdominal haemorrhage. Hepatic tumour should be considered in women presenting with severe upper abdominal pain or liver enlargement (see section 4.3).
Other conditions:
Hypertension
Hypertension increases the risk of stroke and MI (see section 4.4). Although small increases in blood pressure have been reported in many women taking COCs, clinically relevant increases are rare.
If sustained, significant increase in blood pressure occurs, COCs should be discontinued.
COC use is contraindicated in women with uncontrolled hypertension (see section 4.3).
Where considered appropriate, COC use may be resumed if normotensive values can be achieved with antihypertensive therapy.
Gallbladder Disease
COCs may increase the risk of gallbladder disease and may worsen existing disease. For women with symptomatic gallbladder disease, consideration should be given to whether the benefits of COCs outweigh the risks.
Diabetes
Insulin-dependent diabetics without vascular disease can use COCs. However it should be remembered that all diabetics and patients with impaired glucose tolerance are at an increased risk of arterial disease and this should be considered when prescribing COCs. Diabetics with existing vascular disease are contraindicated from using COCs (see section 4.3).
Known hyperlipidaemias
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
Women who are being treated for hyperlipidaemias are at an increased risk for arterial disease and should be followed closely if they elect to use COCs. However, routine screening of women on COCs is not appropriate.
Conditions which deteriorate in pregnancy or during previous COC use The following conditions have been reported to occur or deteriorate with both pregnancy and COC use. Such patients who use COCs should be carefully monitored. Consideration should be given to stopping Ovranette if any of the following occur during use:
• Raised blood pressure
• Jaundice and/or pruritus related to cholestasis
• Herpes gestationis
• Chloasma
• Systemic lupus erythematosus (SLE)
• Severe headaches
• Hereditary angioedema
• Mood changes, including depressed mood
• Or any other condition an individual woman has experienced worsening of during pregnancy or previous use of COCs.
Bleeding Irregularities
Irregular bleeding may occur especially during the first 3 months of COC use. If bleeding irregularities persist or occur after previously regular cycles, then non-hormonal causes should be considered and adequate diagnostic measures are indicated to exclude malignancy or pregnancy.
Some women may encounter post-pill amenorrhea (possibly with anovulation) or oligomenorrhea, especially when such a condition was pre-existent.
Ovranette contains sucrose and lactose
This product contains lactose and sucrose. Patients with rare hereditary problems of galactose intolerance, fructose intolerance, the Lapp lactase deficiency, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take Ovranette.
Interaction with other medicinal products and other forms of interaction
4.5
Interactions between ethinylestradiol (EE) and/or levonorgestrel (LNG) and other substances may lead to decreased or increased serum EE and/or LNG concentrations. The prescribing information of concomitant medications should be consulted to identify potential interactions.
|
SUBSTANCES THAT MAY DECREASE SERUM EE CONCENTRATIONS | |
|
Drugs and Mechanism |
Result & Contraception Management |
|
Hepatic Enzyme Inducers Drugs which induce hepatic microsomal enzymes (especially cytochrome P450 3A4) increase the metabolism of contraceptive steroids and hence may result in decreased serum EE and/or LNG concentrations. Drugs in this category include: Anti-retroviral agents: Ritonavir, nevirapine Anticonvulsants: Carbamazepine, barbiturates, primidone, phenytoin, topiramate, oxcarbazepine Anti-microbials: Rifampicin, rifabutin Griseofulvin (anti-fungal) Anti-inflammatory agents: Phenylbutazone, dexamethasone Stimulants: Modafinil Herbal remedies: St. John’s wort (Hypericum perforatum) |
Increase incidence of breakthrough bleeding and menstrual irregularities. May decrease efficacy of Ovranette therefore, a non-hormonal back-up method (such as condoms and/or spermicide) should be considered in addition to Ovranette use. For short term use: Back up method of contraception such as condoms or spermicide or entire duration of concomitant therapy and 28 days after completion of concomitant therapy (may take several weeks until enzyme induction has completely subsided, depending on the dosage, duration of use, and rate of elimination of the inducing substance). For long term users: In the case of prolonged use of such substances Ovranette should not be considered the primary contraceptive and alternative contraceptives not affected by enzyme-inducing drugs should be considered. |
|
Enterohepatic circulation (EHC) inhibitors: Short-term use of antibiotics has been reported to alter gut flora and reduce the enterohepatic circulation of EE; consequently resulting in decreased EE serum concentrations. Drugs in this category include: Antibiotic agents: Ampicillin and other penicillins, Ttetracyclines |
Short term users: During short term (<3 weeks) concomitant use of antibiotics (except rifampicin) additional contraceptive protection, such as condoms, is recommended during the treatment course and for 7 days after the antibiotic is stopped. Long term users: No additional contraceptive protection is required for women starting COC whom have been using antibiotics long-term (> 3 weeks), unless the antibiotic is changed (in this instance contraception should be managed as for short courses). |
Other drug interactions:
Oral contraceptives may interfere with the metabolism of certain other drugs.
Increased plasma concentrations of ciclosporin and theophylline have been reported with concomitant administration of OCs. COCs have been shown to induce metabolism of lamotrigine resulting in sub-therapeutic plasma concentrations of lamotrigine.
Laboratory tests
The use of contraceptive steroids may influence the results of certain laboratory tests, including biochemical parameters of liver, thyroid, adrenal and renal function, plasma levels of (carrier) proteins (e.g. corticosteroid binding globulin and lipid/lipoprotein fractions), parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. Changes generally remain within the normal laboratory range.
4.6 Fertility, pregnancy and lactation
Pregnancy
Ovranette is not indicated during pregnancy.
In cases of suspected pregnancy, pregnancy should be confirmed promptly before initiating or discontinuing treatment.
Extensive epidemiological studies have revealed neither an increased risk of birth defects in children born to women who used COCs prior to pregnancy, nor a teratogenic effect at unintentional intake of contraceptive pills in early pregnancy.
Breast-feeding
Lactation may be influenced by contraceptive pills as they may reduce the amount of breast milk and change its composition. Thus, the use of combined oral contraceptives should generally not be recommended until the nursing mother has weaned her child off breast milk. Small amounts of the contraceptive steroids and/or their metabolites may be excreted in breast milk. These amounts may affect the child.
4.7 Effects on ability to drive and use machines
Ovranette has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Use of COCs has been associated with increased risk of the following:
• Arterial and venous thrombotic and thromboembolic events, including myocardial infarction, stroke, transient ischemic attack, retinal vein thrombosis, venous thrombosis and pulmonary embolism*
• Cervical intraepithelial neoplasia and cervical cancer*
• Breast cancer diagnosis*
• Benign hepatic tumours (e.g. focal nodular hyperplasia, hepatic adenoma)*
• Conditions reported to deteriorate with pregnancy or previous COC use include: Crohn’s disease, ulcerative colitis, porphyria, systemic lupus erythematosus, herpes gestationis, exacerbation of chorea, cholestatic jaundice
Within the organ system classes, adverse reactions are listed under the headings of frequency (number of patients expected to experience the reaction), using the following categories:
Very common: (>1/10)
Common: (>1/100 to <1/10)
(> 1/1,000 to <1/100)
Uncommon:
Rare:
Very rare: Not known:
(>1/10,000 to <1/1,000)
(<1/10,000)
(Cannot be estimated from the available data)
Infections and Infestations:
Common: Vaginitis, including candidiasis
Neoplasms benign, malignant and unspecified (incl cysts and polyps)
Very Rare: Liver tumours, cervical cancer*, breast cancer*
Immune system disorders
Rare: Hypersensitivity, anaphylactic/anaphylactoid reactions,
Exacerbation of hereditary angioedema*
Very rare: Exacerbation of systemic lupus erythematosus
Metabolism and nutrition disorders
Uncommon: Changes in appetite (increase or decrease)
Rare: Change in glucose tolerance
Very rare: Exacerbation of porphyria
Psychiatric disorders
Common: Mood changes including depressed mood*, nervousness, changes in libido
Nervous system disorders
Very common: Headache including migraines*
Common: Dizziness
Very rare: Exacerbation of chorea
Eye disorders
Rare: Contact lens intolerance
Very rare: Retinal vein thrombosis
Vascular disorders:
Rare: Venous thromboembolic disorders, arterial thromboembolic disorders
Not known: Aggravation of varicose veins
Gastrointestinal disorders
Common: Nausea, vomiting, abdominal pain/cramps
Uncommon: Diarrhoea
Very rare: Pancreatitis
Not known: Crohn’s disease, ulcerative colitis, bloating
Hepato-biliary disorder
Rare: Cholestatic j aundice
Very rare: Gallbladder disease and exacerbation of existing disease*
Skin and subcutaneous tissue disorders
Common: Acne
Uncommon: Rash, urticaria, chloasma which may persist
Rare: Erythema nodosum, erythema multiforme
Reproductive system and breast disorders
Very common: Breakthrough bleeding/spotting*
Common: Breast pain, breast tenderness, breast enlargement, breast secretion
Uncommon: Changes in menstrual flow
Rare: Dysmenorrhea, amenorrhea, vaginal discharge
General disorders and administration site conditions:
Common: Fluid retention/edema
Investigations
Common: Changes in weight (increase or decrease)
Uncommon: Increase in blood pressure*, changes in serum lipid levels, including
hypertriglyceridemia*
* Please refer to section 4.4 for more information.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
4.9. Overdose
Symptoms of oral contraceptive overdosage in adults and children may include nausea, vomiting, breast tenderness, dizziness, abdominal pain, drowsiness/fatigue; vaginal bleeding may occur in females. There is no specific antidote and further treatment of overdose, if necessary, is directed to the symptoms.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Progestogens and estrogens, fixed combinations. ATC code: G03FA11.
Ethinylestradiol is a synthetic oestrogen which has actions and uses similar to those of oestradiol, but is much more potent.
Norgestrel is a progestational agent with actions similar to those of progesterone. It is more potent as an inhibitor of ovulation than norethisterone and has androgenic activity.
5.2 Pharmacokinetic properties
Ethinoestradiol is absorbed by the gastrointestinal tract. It is only slowly metabolised and excreted in the urine.
Norgestrel is absorbed from the gastro-intestinal tract. Metabolites are excreted in the urine and faeces as glucuronide and sulphate conjugates.
5.3 Preclinical safety data
Nothing of relevance to the prescriber
6.
PHARMACEUTICAL PARTICULARS
6.1 List of excipients Core:
Lactose monohydrate Maize starch Povidone 25 Magnesium stearate Talc
Purified water
Coating:
Sucrose
Polyethylene glycol 6000 Calcium carbonate Talc
Povidone 90 Purified water White wax Wax carnauba
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years.
6.4 Special precautions for storage
Do not store above 25°C.
6.5 Nature and Contents of Container
Aluminium foil and PVC blister strip of 21 tablets.
Each blister strip is packaged in an aluminium foil pouch together with a silica gel desiccant sachet.
Cartons containing 1, 3 and 50 blisters.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements for disposal.
7 MARKETING AUTHORISATION HOLDER
Pfizer Limited Ramsgate Road
8
9.
10
Sandwich
Kent
CT13 9NJ United Kingdom
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 26 February 1996 Date of latest renewal: 05 December 2008