Oxpian 60Mg Prolonged-Release Tablets
Package leaflet: Information for the patient
OXPIAN 5 mg, 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg AND 80 mg PROLONGED-RELEASE
TABLETS
oxycodone hydrochloride
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Oxpian is and what it is used for
2. What you need to know before you take Oxpian
3. How to take Oxpian
4. Possible side effects
5. How to store Oxpian
6. Contents of the pack and other information
1. What Oxpian is and what it is used for
Oxpian contains the active ingredient oxycodone hydrochloride, which belongs to a group of medicines called opioids. These are strong painkillers.
Oxpian is used to relieve severe pain, which can only be controlled by opioid analgesics in adults and adolescents 12 years of age and older.
2. What you need to know before you take Oxpian Do not take Oxpian if you:
• are allergic to oxycodone hydrochloride or any of the other ingredients of this medicine (listed in section 6)
• have severe breathing problems, low amounts of oxygen in your blood (hypoxia) or too much carbon dioxide in your blood
• suffer from severe chronic obstructive lung disease, cor pulmonale (cardiac changes due to chronic overload of lung circulation) or acute, severe bronchial asthma
• suffer from intestinal paralysis
• have a sudden, severe abdominal pain or suffer from a delayed gastric emptying.
Warnings and precautions
Talk to your doctor or pharmacist before taking Oxpian if you:
• are older or debilitated
• have lung, liver or kidney problems
• suffer from certain illnesses of the thyroid gland, impaired function of the thyroid gland
• suffer from adrenal insufficiency (Addison’s disease)
• suffer from enlargement of the prostate
• suffer from alcoholism or are undergoing alcohol withdrawal
• suffer from known opioid-dependence
• suffer from inflammation of the pancreas
• have conditions with increased brain pressure such as head injury
• suffer from disturbances of circulatory regulation
• suffer from colic of the bile duct and ureter
• suffer from low blood pressure or reduced blood volume
• suffer from epilepsy or have a seizure tendency
• take monoamine oxidase inhibitors, also referred to as MAOIs (for the treatment of depression)
• suffer from an inflammatory bowel disorder
• have recently had abdominal surgery.
Please talk to your doctor if any of these apply to you or if any of these conditions applied to you in the past.
When used for a long time, your body may develop tolerance to the effects of this medicine and as a result, progressively higher doses may be required to control the pain.
Chronic use of Oxpian may lead to physical dependence and a withdrawal syndrome may occur when you stop taking it suddenly. When you no longer require therapy with oxycodone hydrochloride, it may be advisable to reduce the dose gradually to prevent symptoms of withdrawal.
When used as directed in patients suffering from chronic pain, the risk of developing physical or psychological dependence is significantly reduced and needs to be weighed against the potential benefit of taking this medicine. Please discuss this with your doctor.
Increased sensitivity to pain that does not respond to dose increases can rarely develop. If this happens, your doctor will reduce your dose or switch you to an alternative opioid painkiller.
Oxpian is not recommended for use before an operation or in the 24 hours after an operation.
Oxpian should be used with particular care in patients with a history of or present alcohol and drug abuse.
Children and adolescents
The effects of oxycodone have not been investigated in children under 12 years. Safety and efficacy have not been established and therefore use in children under 12 years of age is not recommended.
Older patients
If kidney or liver function is not impaired, a dose adjustment is usually not necessary for elderly patients. Other medicines and Oxpian
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The following medicines may influence the effect or side effects of Oxpian;
• sleeping pills or tranquillizers (sedatives, hypnotics)
• anti-depressants
• anaesthetics
• muscle relaxants
• other opioids or alcohol can enhance the side effects of oxycodone, in particular depressed breathing (respiratory depression).
• other medicines that act against parasympathetic and cholinergic nerve fibres on the central nervous system
• medicines used to treat allergies (antihistamines)
• medicines used to treat vomiting (antiemetics)
• medicines used to treat Parkinson’s disease can enhance certain side effects of oxycodone (e.g. constipation, dry mouth or urinary disturbances)
• anticoagulants of the coumarin type (medicines used to reduce blood clotting)
• monoamine oxidase inhibitors (MAOIs) such as moclobemide, phenelzine, isoniazid, tranylcypromine or selegiline as these can enhance some side effects of oxycodone (e.g. excitation, decrease or increase in blood pressure).
The following medicines may possibly increase the blood levels of oxycodone and your doctor may need to re-consider the dose of Oxpian:
• medicines used to treat infections (e.g. clarithromycin, erythromycin and telithromycin) or to treat fungal infections (e.g. ketoconazole, voriconazole, itraconazole, and posaconazole)
• medicines such as rifampicin (used to treat tuberculosis)
• medicines used to treat HIV (e.g. boceprevir, ritonavir, indinavir, nelfinavir and saquinavir)
• cimetidine (to treat heart burn)
• medicines such as paroxetine and fluoxetine (antidepressants) and St John’s wort (herbal medicine)
• quinidine (used in the treatment of heart diseases)
• carbamazepine, phenytoin (used to treat epilepsy).
Grapefruit juice may also increase the blood levels of oxycodone.
Oxpian with food, drink and alcohol
You should NOT drink alcohol while you are taking Oxpian. Drinking alcohol whilst taking Oxpian may make you feel more sleepy or increase the risk of serious side effects such as shallow breathing with a risk of stopping breathing, and loss of consciousness.
Grapefruit juice can increase the effect of oxycodone. Therefore, you should avoid drinking grapefruit juice while taking Oxpian.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
There are limited data from the use of oxycodone in pregnant women. Oxycodone passes through the placenta into the blood circulation of the baby.
Use of oxycodone during pregnancy may cause withdrawal symptoms in newborns. Infants born to mothers who have received oxycodone during the last 3-4 weeks before labour should be monitored for respiratory depression. Use of oxycodone during childbirth can cause severe breathing difficulties in the newborn. Oxpian should only be used during pregnancy if the benefit outweighs the possible risks to the baby.
Breast-feeding
Oxycodone may pass into breast milk and may cause breathing difficulties in the newborn. Oxpian should therefore not be used during breast-feeding.
Driving and using machines
Oxycodone may affect your ability to drive and use machines.
With stable therapy, a general ban on driving a vehicle may not be necessary. The treating physician must assess the individual situation. Please discuss with your doctor whether or under what conditions you can drive a vehicle.
Oxpian contains lactose
This medicinal product contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
3. How to take Oxpian
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Adults and adolescents (12 years of age and older)
The recommended initial dose is 5 or 10 mg oxycodone hydrochloride, twice a day (every 12 hours).
However, your doctor will prescribe the dose required to treat pain.
Further determination of the daily dose, the division into the single doses and any dose adjustments during the further course of therapy are performed by the treating physician and depend on the previous dosage.
Patients who have already taken opioids can start treatment with higher dosages taking into account their experience with opioid treatment.
Some patients who receive Oxpian according to a fixed schedule need rapidly acting painkillers as rescue medication to control breakthrough pain. Oxpian is not intended for the treatment of breakthrough pain.
For the treatment of non-cancer pain, a daily dose of 40 mg of oxycodone hydrochloride (20 mg given twice a day) is generally sufficient, but higher doses may be necessary. Patients with cancer pain usually require doses from 80 to 120 mg of oxycodone hydrochloride which may be increased up to 400 mg in individual cases.
The treatment needs to be controlled regularly with regard to pain relief and other effects in order to achieve the best pain therapy possible, as well as to be able to treat any occurring side effects efficiently and to decide whether treatment should be continued.
Kidney/liver impairment or low body weight
If you have impaired kidney and/or liver function or if you have a low body weight, your doctor may prescribe a lower starting dose.
Method and duration of administration
Swallow the prolonged-release tablets whole with a sufficient amount of liquid (% glass of water) with or without food in the morning and in the evening following a fixed schedule (e.g. at 8 a.m. and 8 p.m.).
The tablets must not be broken, crushed or chewed as this leads to rapid oxycodone release due to the damage of the prolonged release properties. The administration of broken, chewed or crushed Oxpian leads to a rapid release and absorption of a potentially fatal dose of oxycodone (see section “If you take more Oxpian than you should”).
Oxpian are for oral use only. In case of abusive injection (injection in a vein), the inactive ingredients in the tablet may cause destruction (necrosis) of the local tissue, change of lung tissue (granulomas of the lung) or other serious, potentially fatal events.
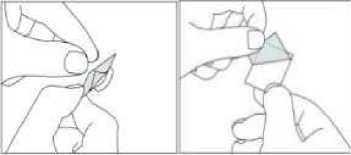
[For child resistant blister packs only:]
Instructions for use of child resistant blisters:
1. Do not push the tablet directly out of the pocket.
2. Separate one blister cell from the strip at the perforations

3. Carefully peel off the backing to open the pocket.
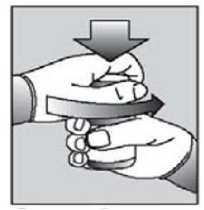
[Instructions for use of child resistant HDPE containers:] Push down the lid and turn to open
Your doctor will adjust the dosage depending on the pain intensity and how you respond to the treatment.
Take the number of prolonged-release tablets determined by your doctor twice daily.
If you take more Oxpian than you should
If you have taken more Oxpian than prescribed, you should inform your doctor or your local poison control centre immediately.
The following symptoms may occur: constricted pupils, depressed breathing, floppy muscles and drop in blood pressure. In severe cases, circulatory collapse, mental and motor inactivity, unconsciousness, slowing of the heart rate, accumulation of water in the lungs, low blood pressure and death may occur; abuse of high doses of strong opioids such as oxycodone can be fatal. If you have taken too much Oxpian, DO NOT expose yourself to situations requiring elevated concentration e.g. driving a car.
If you forget to take Oxpian
If you use a smaller dose of Oxpian than directed or you miss the intake of the tablets, pain relief will consequently be insufficient or stop altogether.
You can make up for a forgotten tablet if the next regular intake is not due for at least another 8 hours. You can then continue to take the tablets as directed.
You should also take the prolonged-release tablets if the time to the regular next intake is shorter, but postpone the next intake by 8 hours. In principle, you should not take Oxpian more than once every 8 hours.
Do not take a double dose to make up for a forgotten tablet.
If you stop taking Oxpian
Do not stop treatment without informing your doctor.
When a patient no longer requires therapy with Oxpian, it may be advisable to reduce the dose gradually to prevent symptoms of withdrawal (see section 2, “What you need to know before you take Oxpian”).
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following side effects, stop taking Oxpian and contact your doctor immediately.
• depressed (shallow) breathing, especially in elderly or debilitated patients, which can cause severe drop in blood pressure
• constricted pupils
• sudden constriction of the airways, causing difficulty in breathing (bronchospasm)
• abdominal cramps
• suppression of cough reflex.
Other possible side effects
Very common (may affect more than 1 in 10 people):
• sedation (tiredness to drowsiness) - this is most likely when you start taking your tablets or when your dose is increased, but it should wear off after a few days.
• dizziness
• headache
• constipation
• feeling sick (nausea)
• being sick (vomiting)
• itching.
Common (may affect up to 1 in 10 people):
• feeling weak (asthenia)
• several psychological side effects such as
• changes in mood (e.g. anxiety, depression)
• changes in activity (nervousness and insomnia)
• changes in performance (abnormal thinking, confusion, amnesia, isolated cases of speech disorders)
• involuntary trembling or shaking
• depressed breathing
• difficulty in breathing or wheezing
• dry mouth, rarely accompanied by thirst; gastrointestinal disorders such as stomach pain; diarrhoea; upset stomach; loss of appetite
• skin disorders such as rash, rarely increased sensitivity to light (photosensitivity), in isolated cases itchy or scaly rash, excessive sweating
• urinary disorders (frequent urination).
Uncommon (may affect up to 1 in 100 people):
• allergic reactions
• dehydration
• agitation
• change in perception such as emotional instability, depersonalisation, a feeling of extreme happiness, hallucinations, change in taste, visual disturbances, abnormally acute sense of hearing, feeling of dizziness or spinning, decreased sex drive; drug dependence (see section 2, “What you need to know before you take Oxpian”)
• abnormal production of antidiuretic hormone
• loss of memory, fits, increased tightness and difficulty in stretching muscles, both increased and decreased muscle tone; tics; reduced sense of touch; coordination disturbances; speech disorders; fainting; tingling or pins and needles
• feeling unwell, accelerated pulse, being aware of the heart beat
• widening of the blood vessels
• increased coughing; pharyngitis; runny nose; voice changes; difficulty breathing
• oral ulcers; inflammation of the gums, inflamed mouth; difficulty swallowing, wind, flatulence, intestinal obstruction
• increased liver enzymes
• dry skin
• difficulty in passing urine
• disturbances of sexual function, impotence
• injuries due to accidents
• pain (e.g. chest pain); excessive fluid in the tissues (oedema); chills; thirst; migraine; physical dependence with withdrawal symptoms
• changes in tear secretion, constriction of the pupil, visual impairment.
Rare (may affect up to 1 in 1,000 people):
• lymph node disease
• lowering of blood pressure, dizziness when standing up from a sitting or lying position
• muscle spasms (involuntary contraction of the muscle)
• gum bleeding; increased appetite; tarry stool; tooth staining
• herpes simplex (disorder of the skin and mucosa)
• itchy skin rash (hives)
• blood in urine
• changes in body weight (loss or rise); cellulitis.
Not known (frequency cannot be estimated from the available data):
• severe hypersensitivity reactions (anaphylactic reactions)
• aggression
• increased sensitivity to pain which cannot be improved by increasing the dose
• tooth decay
• pain on the right side of the abdomen, itchiness and jaundice caused by inflammation of the gall bladder.
• absence of menstrual bleeding.
Counteractive measures
If you observe any of the above listed side effects, your doctor will usually take appropriate measures.
Constipation may be prevented by fibre enriched diet and increased intake of fluids.
If you are suffering from sickness or vomiting your doctor may prescribe you an appropriate medicine.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Oxpian
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister, carton and container after “EXP”. The expiry date refers to the last day of that month.
Blister packs: Do not store above 25°C.
HDPE container: This medicinal product does not require any special storage conditions.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Oxpian contains
• The active substance is oxycodone hydrochloride. Each prolonged-release tablet contains 5, 10, 15, 20, 30, 40, 60 or 80 mg oxycodone hydrochloride.
• The other ingredients are:
Tablet core: Lactose monohydrate (see section 2 Oxpian contains lactose), hypromellose, povidone, stearic acid, magnesium stearate, colloidal anhydrous silica.
Tablet coating:
5 mg tablets: Polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, Indigo Carmine Aluminium Lake (E132), yellow iron oxide (E172).
10 mg tablets: Titanium dioxide (E171), hypromellose, macrogol, polysorbate 80.
15 mg tablets: Polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, yellow iron oxide (E172), black iron oxide (E172).
20 mg tablets: Polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, red iron oxide (E172).
30 mg tablets: Polyvinyl alcohol, macrogol, talc, red iron oxide (E172), black iron oxide (E172), Indigo Carmine Aluminium Lake (E132)
40 mg tablets: Polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, yellow iron oxide (E172).
60 mg tablets: Polyvinyl alcohol, macrogol, talc, red iron oxide (E172), carmine (E120), black iron oxide (E172).
80 mg tablets: Polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, Indigo Carmine Aluminium Lake (E132), yellow iron oxide (E172).
What Oxpian looks like and contents of the pack
Oxpian 5 mg prolonged-release tablets are blue, round, biconvex tablets, 7 mm in diameter, with ‘OX 5’debossed on one side.
Oxpian 10 mg prolonged-release tablets are white, round, biconvex tablets, 9 mm in diameter, with ‘OX 10’debossed on one side.
Oxpian 15 mg prolonged-release tablets are grey, round, biconvex tablets, 9 mm in diameter, with ‘OX 15’debossed on one side.
Oxpian 20 mg prolonged-release tablets are pink, round, biconvex tablets, 7 mm in diameter, with ‘OX 20’debossed on one side.
Oxpian 30 mg prolonged-release tablets are brown, round, biconvex tablets, 9 mm in diameter, with ‘OX 30’debossed on one side.
Oxpian 40 mg prolonged-release tablets are yellow, round, biconvex tablets, 7 mm in diameter, with ‘OX 40’debossed on one side.
Oxpian 60 mg prolonged-release tablets are red, round, biconvex tablets, 9 mm in diameter, with ‘OX 60’debossed on one side.
Oxpian 80 mg prolonged-release tablets are green, round, biconvex tablets, 9 mm in diameter, with ‘OX 80’debossed on one side.
Oxpian are available in child-resistant blister packs (PVC/Al/PET/paper) and non-child-resistant blister packs (PVC/Aluminium) of:
5 mg: 14, 25, 28, 30, 56, 60, 98, 100 prolonged-release tablets.
10 mg, 20 mg, 40 mg, 80 mg: 10, 14, 25, 28, 30, 50, 56, 60, 98, 100 prolonged-release tablets.
15 mg: 28, 30, 56, 98,100 prolonged-release tablets.
30 mg, 60 mg: 10, 30, 56, 60, 100 prolonged-release tablets.
Oxpian are also available in white, round, child-resistant, HDPE tablet containers with LDPE caps containing:
5 mg, 10 mg: 98 and 100 prolonged-release tablets.
15 mg, 60 mg: 100 prolonged-release tablets.
20mg, 40 mg, 80 mg: 50 and 100 prolonged-release tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
TEVA UK Limited, Eastbourne, BN22 9AG, UK
Manufacturer
Actavis ehf., Reykjavikurvegur 78, IS- 220 Hafnarfjordur, Iceland *OR
Actavis UK Limited, Whiddon Valley, Barnstaple, North Devon, EX32 8NS, United Kingdom *OR
Balkanpharma-Dupnitsa AD, 3 Samokovsko Shosse Str., Dupnitsa 2600, Bulgaria *OR
Teva Operations Poland Sp. z.o.o, ul. Mogilska 80. , Krakow, 31-546, Poland *OR
Merckle GmbH, Ludwig-Merckle-StraBe 3, Blaubeuren, 89143, Germany This leaflet was last revised in 06/2016
Only the actual site of batch release will appear on the printed version of the leaflet