Paclitaxel 6Mg/Ml Concentrate For Solution For Infusion
(Travesh Sharma) D:\Europe\Paclitaxel (Bordan)\Ireland-UK\15 July 2016\Paclitaxel - Package Insert - Ireland+UK (Bordon).indd Size: 339 x 280 mm
Package leaflet: information for the user
Paclitaxel 6 mg/ml
concentrate for solution for infusion
Paclitaxel
The name of your medicine is ‘Paclitaxel 6 mg/ml concentrate for solution for infusion’ but in the rest of the leaflet it will be called “Paclitaxel”.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Paclitaxel is and what it is used for
2. What you need to know before you use Paclitaxel
3. How Paclitaxel is given to you
4. Possible side effects
5. How to store Paclitaxel
6. Contents of the pack and other information
1. What Paclitaxel is and what it is used for
Paclitaxel belongs to a group of anti-cancer medicines called taxanes. These agents inhibit the growth of cancer cells.
Paclitaxel is used to treat:
Ovarian cancer:
• as first-line therapy (after initial surgery in combination with the platinum-containing medicine cisplatin).
• after standard platinum-containing medicines have been tried but did not work.
Breast cancer:
• as first-line therapy for advanced disease or disease which has spread to other parts of the body (metastatic disease). Paclitaxel is either combined with an anthracycline (e.g. doxorubicin) or with a medicine called trastuzumab (for patients for whom anthracycline is not suitable and whose cancer cells have a protein on their surface called HER 2, see package leaflet of trastuzumab).
• as an additional treatment with anthracycline and cyclophosphamide (AC).
• as a second-line treatment for patients who have not responded to standard treatments using anthracyclines, or for whom such treatment should not be used.
Advanced non-small-cell lung cancer:
• in combination with cisplatin, when surgery and/or radiation therapy aren’t suitable. AIDS-related Kaposi’s sarcoma:
• where another treatment (i.e. liposomal anthracyclines) has been tried but did not work.
2. What you need to know before you use Paclitaxel You should not be given Paclitaxel:
- if you are allergic to paclitaxel or any of the other ingredients of this medicine (listed in section 6), especially polyoxyethylated castor oil (macrogolglycerol ricinoleate).
- if you are breast-feeding;
- if you have too few white blood cells count (baseline neutrophil counts <1.5 x 109/l or <1.0 x 109/l for Kaposi’s sarcoma patients - your doctor will advise you on this) in your blood. Your doctor will take blood samples to check this.
- if you have a serious and uncontrolled infections (only in case paclitaxel is used to treat Kaposi’s sarcoma.
If any of these apply to you, talk to your doctor before starting treatment with Paclitaxel. Paclitaxel is not recommended for use in children (under 18 years).
Warnings and precautions
Talk to your doctor before using Paclitaxel
To minimize allergic reactions, you will be given other medicines before you receive
Paclitaxel.
- If you experience allergic reactions (for example difficulty breathing, shortness of breath, chest tightness, drop in blood pressure, dizziness, light headedness, skin reactions such as rash or swelling).
- If you have fever, severe chills, sore throat or mouth ulcers (signs of bone marrow suppression)
- If you have numbness, tingling, pricking sensations, sensitivity to touch, or weakness of the arms and legs (signs of peripheral neuropathy); a dose reduction of Paclitaxel may be necessary.
- if you have severe liver problems; in that case the use of Paclitaxel is not recommended.
- If you have heart conduction problems.
- If you develop severe or persistent diarrhoea, with fever and stomach pain, during or shortly after the treatment with Paclitaxel. Your colon could be inflamed (pseudomembranous colitis).
- If you had previous radiation to your chest (because it may increase the risk of lung inflammation).
- If you have a sore or red mouth (signs of mucositis) and are treated for Kaposi’s Sarcoma. You may need a lower dose.
This medicinal product contains 49.7 vol % ethanol (alcohol), i.e. up to 23 g per dose, equivalent
to approximately 600 ml beer, approximately 250 ml wine per dose.
Harmful for those suffering from alcoholism.
To be taken into account and high risk groups such as patients with liver disease or epilepsy.
The amount of alcohol in this medicinal product may alter the effects of other medicines.
3. How Paclitaxel is given to you
• To minimise allergic reactions, you will be given other medicines before starting Paclitaxel. These medicines can be given as either tablets or infusion into a vein or both.
• You will receive paclitaxel as a drip into one of your veins (by intravenous infusion), through an in-line filter. Paclitaxel will be administered to you by a healthcare professional. He or she will prepare the solution for infusion before it is given to you. The dose you receive will also depend on results of your blood tests. Depending on the type and severity of the cancer you will receive Paclitaxel either alone or in combination with another anticancer agent.
• Paclitaxel should always be administered into one of your veins over a period of 3 or 24 hours. It is usually given every 2 or 3 weeks, unless your doctor decides otherwise. Your doctor will inform you about the number of courses of Paclitaxel you need to receive.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
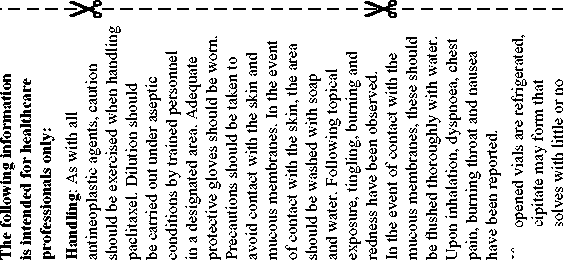
c Ph t:
o. o
S e
C cn
S S- •-»
fi
Given the possibility of extravasation, it is advisable to closely monitor the infusion site for possible infiltration during drug administration.
Tell your doctor immediately if any of these apply to you.
Paclitaxel should always be administered into veins. Administration of Paclitaxel in the arteries can cause inflammation of the arteries, and you can suffer from pain, swelling, redness and heat.
Other medicines and Paclitaxel
Tell your doctor if you are using or have recently used or might use any other medicines. Interaction means that different medicines may influence each other. Interaction may occur and your doctor needs to know when using Paclitaxel together with:
• cisplatin (to treat cancer): Paclitaxel must be given before cisplatin. Your renal function may need to be checked more frequently.
• doxorubicin (to treat cancer): Paclitaxel must be administered 24 hours after doxorubicin, to avoid high level of doxorubicine in your body.
• efavirenz, nevirapine, ritonavir, nelfinavir, or other protease-inhibitors, which are HIV treatments. A dose adjustment of Paclitaxel may be necessary.
• erythromycin, an antibiotic, fluoxetine, an antidepressant or gemfibrozil, used for lowering cholesterol. A dose reduction of Paclitaxel may be necessary.
• rifampicin, an antibiotic used for tuberculosis, carbamazepine, phenytoin or phenobarbital, used for epilepsy. A dose increase of Paclitaxel may be necessary.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant or think you may be pregnant before receiving treatment with Paclitaxel. If there is a chance that you could become pregnant, use an effective and safe method of contraception during treatment. Paclitaxel should not be used during pregnancy unless clearly necessary. Female and male patients of fertile age, and/or their partners should use contraception for at least 6 months after treatment with paclitaxel. Male patients should seek advice regarding cryoconservation of sperm prior to treatment with paclitaxel because of the possibility of irreversible infertility.
If you are breast-feeding, tell your doctor. It is not known if paclitaxel passes into breast milk. Because of the possibility of harm to the infant stop breast-feeding if you are taking Paclitaxel. Do not restart breast-feeding unless your doctor has allowed you to.
Driving and using machines
There is no reason why you cannot continue driving between courses of Paclitaxel but you should remember that this medicine contains some alcohol and it may be unwise to drive or use machines immediately after a course of treatment due to possible effects on your central nervous system. As in all cases, you should not drive or use machines if you feel dizzy or light-headed. Paclitaxel contains castor oil that may cause severe allergic reactions. If you are allergic to castor oil, talk to your doctor before you receive Paclitaxel.
Paclitaxel contains castor oil (macrogolglycerol ricinolate) and alcohol
Paclitaxel contains castor oil that may cause severe allergic reactions. If you are allergic to castor oil, talk to your doctor before you receive Paclitaxel.
x-
x fi .
<'J X 1— <J fi. X
•9 P 2
fi ^
Oh
X X) S M * fi
00
G £ G £
Q_ ^
O.
£ O
Q > • u
o
5 b
6 1
> .s
Js |
= V
© C5
& a? - •-
§• S
j- ■= Pm «
_3
3
8 3 3 's o 2
sS °
•n g
•3
2 3 8 fi
w
fi o a fc, S
X fi X
8 .2 e '? 3 » £ o
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you notice any signs of allergic reactions. These may include one or more of the following:
• flushing,
• skin reactions,
• itching,
• chest tightness,
• shortness or difficulty in breathing,
• swelling.
These can all be signs of serious side effects.
Tell your doctor immediately if you experience;
• fever, severe chills, sore throat or mouth ulcers (signs of bone marrow suppression).
• numbness or weakness of the arms and legs (signs of peripheral neuropathy).
• severe or persistent diarrhoea, with fever and stomach pain.
Very common: may affect more than 1 in 10 people
• Minor allergic reactions such as flushing, rash, itching
• Infections: mainly upper respiratory infection, urinary tract infection
• Sore throat or mouth ulcers, sore and red mouth, diarrhoea, feeling or being sick (nausea, vomiting)
• Hair loss
• Pain in the muscles, cramps, pain in the joints
• Numbness, tingling or weakness in arms and legs (all symptoms of peripheral neuropathy)
• Tests may show: reduction of blood platelet ount which can lead to bleeding an bruising more easily than normal, white or red blood cells count, low blood pressure
Common: may affect up to 1 in 10 people
• Temporary mild nail change and skin changes, reactions at injection sites (localised swelling, pain, and redness of the skin)
• Tests may show: slower heart rate, severe elevation in liver enzymes (alkaline phosphatase and AST - SGOT)
Uncommon: may affect up to 1 in 100 people
• Shock due to infections (known as ‘septic shock’)
• Palpitations, cardiac dysfunction (AV block, cardiomyopathy), rapid beating of the heart, heart attack, respiratory distress
• Fatigue, sweating, fainting (syncope), significant allergic reactions, phlebitis (inflammation of a vein), swelling of the face, lips, mouth, tongue or throat
• Back pain, chest pain, pain around hands and feet, chills, abdominal (tummy) pain
• Tests may show: severe elevation of bilirubin (jaundice), high blood pressure, and blood clot.
>«g
fi 22 .2 O
fi xo X ox O i/~>
<U X
8 o
3 {J’S fi •fi o o o
</"1 05 05 05

OJ)
.fi fi
g fi
_c- X ©3 *7? —
X ^
X 2 X fi
o
> -fi
O M 2
<N
<N
O .g
VI c/
§ ” H 05
& Cl
•fi fi
o
> 'Z X .tfi
(Travesh Sharma) D:\Europe\Paclitaxel (Bordan)\Ireland-UK\15 July 2016\Paclitaxel - Package Insert - Ireland+UK (Bordon).indd Size: 339 x 280 mm
Rare: may affect up to 1 in 1,000 people
• Shortage of white blood cells with fever and increased risk of infection (febrile neutropenia)
• Affection of nerves with feeling of weakness in muscles of arms and legs (motor neuropathy)
• Heart failure (Cardiac failure)
• Shortness of breath, pulmonary embolism, lung fibrosis, interstitial pneumonia, dyspnoea, pleural effusion
• Bowel obstruction, bowel perforation, inflammation of colon (ischaemic colitis), inflammation of the pancreas (pancreatitis)
• Pruritus, rash, skin redness (erythema)
• Blood poisoning (sepsis), peritonitis pneumonia
• Pyrexia, dehydration, asthenia, oedema, malaise
• Serious and potentially fatal hypersensitivity reactions (anaphylactic reactions)
• Tests may show: increase in blood creatinine indicating renal function impairment Very rare: may affect up to 1 in 10,000 people
• Irregular rapid heart rhythm (atrial fibrillation, supraventricular tachycardia)
• Sudden disorder in blood forming cells (acute myeloid leukaemia, myelodysplastic syndrome)
• Optic nerve and/or visual disturbances (scintillating scotomata)
• Hearing loss or reduction (ototoxicity), ringing in the ears (tinnitus), vertigo
• Cough
• Blood clot in a blood vessel of abdomen and bowel (mesenteric thrombosis), inflammation of colon sometimes with persistent severe diarrhoea (pseudomembranous colitis, neutropenic colitis), dropsy (ascites), oesophagitis, constipation.
• Serious hypersensitivity reactions including fever, skin redness, pain in joints and/or inflammation of the eye (Stevens-Johnson syndrome), local peeling of the skin (epidermal necrolysis), redness with irregular red (exudative) spots (erythema multiforme), inflammation of the skin with blisters and peeling (exfoliative dermatitis), urticaria, loose nails (patients on therapy should wear sun protection on hands and feet).
• Loss of appetite (anorexia).
• Serious and potentially fatal hypersensitivity reactions with shock (anaphylactic shock).
• Disturbed liver function (hepatic necrosis, hepatic encephalopathy (both with reported cases of fatal outcome))
• Confusional state.
• Grand mal seizures, brain nerve disorder (autonomic neuropathy; affection of the involuntary body functions, this can result in ileus and low blood pressure), convulsions, brain disease (encephalopathy), dizziness, headache, problems with coordination (ataxia)
Not known: frequency cannot be estimated from the available data
• Rapid destruction of tumors (tumor lysis syndrome)
• Fluid collection in the macula of the eye (Macular edema), presence of perceived flashes of light in the eye (photopsia), deposits within the eye's vitreous humour (vitreous floaters)
• Inflammation of the veins (Phlebitis)
• Thickening and hardening of the skin as well as the blood vessels and the internal organs (Scleroderma)
• “Butterfly rash” (Systemic lupus erythematous)
• Coagulation disorders (disseminated intravascular coagulation)
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. By reporting side effects you can help provide more information on the safety of this medicine.
For UK - You can also report side effects directly via the Yellow Card Scheme at: www.mhra.
gov.uk/yellowcard
For Ireland -
Earlsfort Terrace
Dublin 2
Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie e-mail: medsafety@hpra.ie
5. How to store Paclitaxel
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Keep the vial in the outer carton in order to protect from light.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of that month.
Do not use this medicine if you notice cloudy solution or an insoluble precipitate.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Paclitaxel contains
- The active substance is paclitaxel . Each ml of concentrate for solution for infusion contains 6 mg of paclitaxel.
One 5 ml vial contains 30 mg paclitaxel.
One 16.7 ml vial contains 100 mg paclitaxel.
One 25 ml vial contains 150 mg paclitaxel.
One 50 ml vial contains 300 mg paclitaxel.
One 100 ml vial contains 600 mg paclitaxel.
- The other ingredients are ethanol anhydrous, macrogolglycerol ricinoleate and citric acid anhydrous (for pH adjustment)
What Paclitaxel looks like and contents of the pack
Concentrate for solution for infusion.
Paclitaxel is a clear, slightly yellowish solution.
Paclitaxel is available in glass vials. The glass vials are sealed with Teflon® coated rubber stoppers.
Pack sizes: Packs containing 1 or 5 glass vials.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Fresenius Kabi Limited Cestrian Court, Eastgate Way,
Manor Park, Runcorn,
Cheshire, WA7 1NT UK
Manufacturer
Fresenius Kabi Oncology Plc.
Lion Court, Farnham Road, Bordon,
Hampshire, GU35 0NF, United Kingdom
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Austria |
Paclitaxel Kabi 6 mg/ml Konzentrat zur Herstellung einer Infusionslosung |
|
Belgium |
Paclitaxel Fresenius Kabi |
|
Bulgaria |
Paclitaxel Kabi 6 mg/ml KoH^HTpaT 3a HH$y3HOHeH pa3TB0p |
|
Czech Republic |
Paclitaxel Kabi 6 mg/ml koncentrat pro pnpravuinfuzmho roztoku |
|
Denmark |
Paclitaxel Fresenius Kabi 6 mg/ koncentrat til infusionsvaeske, oplosning |
|
Estonia |
Paclitaxel Kabi 6 mg/ml infusioonilahuse kontsentraat |
|
Germany |
Paclitaxel Kabi 6 mg/ml Konzentrat zur Herstellung einer Infusionslosung |
|
Finland |
Paclitaxel Fresenius Kabi 6 mg/ml infuusiokonsentraatti, liuosta varten |
|
France |
Paclitaxel Kabi 6 mg/ml solution a diluer pour perfusion |
|
Hungary |
Paclitaxel Kabi 6 mg/ml koncentratum oldatos infuziohoz |
|
Ireland |
Paclitaxel 6 mg/ml concentrate for solution for infusion |
|
Italy |
Paclitaxel Kabi 6 mg/ml concentrato per soluzione per infusione |
|
Latvia |
Paclitaxel Kabi 6 mg/ml koncentrats infuziju sklduma pagatavosanai |
|
Lithuania |
Paclitaxel Kabi 6 mg/ml koncentratas infuziniam tirpalui |
|
Luxemburg |
Paclitaxel Kabi 6 mg/ml Konzentrat zur Herstellung einer Infusionslosung |
|
Netherlands |
Paclitaxel Fresenius Kabi |
|
Norway |
Paclitaxel Fresenius Kabi 6 mg/ml konsentrat til infusjonsv^ske |
|
Poland |
Paclitaxel Kabi |
|
Portugal |
Paclitaxel Kabi 6 mg/ml concentrado para solu9ao para perfusao |
|
Romania |
Paclitaxel Kabi 6 mg/ml concentrat pentru solutie perfuza |
|
Slovakia |
Paclitaxel Kabi 6 mg/ml |
|
Slovenia |
Paklitaksel Kabi 6 mg/ml koncentrat za raztopino za infundiranje |
|
Spain |
Paclitaxel Fresenius Kabi 6 mg/ml concentrado para solucion para perfusion |
|
Sweden |
Paclitaxel Fresenius Kabi 6 mg/ml koncentrat till infusionsvatska, losning |
|
United Kingdom |
Paclitaxel 6 mg/ml concentrate for solution for infusion |
This leaflet was last revised in 07/2016.
G
<4-1 G o ©
© £ § I
© G
© m
B x
X G
©J) O
G
O
X .ti Cl
G
.2 ©
©
X O -
0 X
* 3 ^
Cl s-C/3 G G
z 3 .2
u 3
_r © X
1 a. o
-2 3 5/2
"2 © © © X 3 3 ^ p
X X G O y
Cl © O ©
■ S5* t/5 • frt ^3
, •- « © X X > . X Cl X X >
.2 Vi
UiJ r-
3 2
c
T3 &
^ w
3 Q
x © 2 ° 5 s-X Cl
©J)
/
X > * © X X
, ^, — ,. © s-
G Cl ^ © .2
SS/ L- *-L C* © .3
8 2 S S-
CL4
X
X
fi <4-,
w o
3. ©
_C| C/3
4-> ^
w ^
i w W) 1 X
G
<3
rs5
Cl O o X o. I
>«g
x __
i— •—)
3 o S o " -S ^
!» >—i <r
T3 ©0 ffi
X
.9 o
x Q
8 g .
© /
2-x
X C/3
H 8 GO J
^ X
a, ^ o 2
3 >> ^
G G G © © © Is X. *3
x
2 ’X
© Hi
G
© r-
— G
©J)
x © x
Cl X .52 X ~o X
© £
£ “ X ©
-1 § 2 T3