Panadol 1000Mg Tablets
E
E
DO

E
E
«/) ■I—•
cd

CD
u
CC
CO
k\3 o not store above 25°C.
Panadol is a registered trade mark of the GlaxoSmithKline group of companies.
^GlaxoSmithKline
■innnnnniinnnnnn"
\\GlaxoSmithKline Consumer Healthcare, ^Brentford,TW8 9GS.U.K.
XX 00000/0000

w» ®'3
|
E |
E | |
|
E |
E |
E |
|
in |
E |
in |
|
CN |
9 |
r>i |
■D
.E*
1
<DO®
^xWWWWWWWWWWWv

Do not store above 25°C.
Panadol is a registered trade mark of the GlaxoSmithKline group of companies.
\
GlaxoSmithKline Consumer Healthcare, Brentford, TW8 9GS,U.K.
XX 00000/0000
GlaxoSmithKline

E
E
: oi* IS .2 ,
IB O
tSS-a
(U A m '<
1 S ■■
u _u .
c d- +i £ £ tv
155 oLS
Irt Q_
E ®
O' uj >»
,E S n ■E tj — a; co c ® f ar «
tea-®* o
IS
fO ® — 10 £ 9- ® +-
=> Z If
■s S
® . 3
+-< Jr
! CO C ® -c S
o o O £ Q Q

fill
0 0^0 ■O c w c CO £ re f= c «* +j co
0-030




<
O

E
E


Schawk, Kingsway North, Newcastle, Tyne t Wear NE11 OIH, UK T+44(0) 191 491 7777 F +44 (9) 191 487 9973 V2 0BNov20D8
E
E
m
E
E
m


GlaxoSmithKline
GSK Drawing No.: L655D001/02 Dimensions: 45 x 153 mm & 45x466 mm
Factory: Catalent
Component: Fix-A-Form Label
Date : 06th January 2009
Profile Keys:

Copy/Text Free Pharma Code Patch Release Varnish
2mm
29mm
12mm
2mm
CONTAINS PARACETAMOL. Do not take with any other paracetamol-containing products
Immediate medical advice should be sought in the event of an overdose, even if you feel well.
o
E
_Q
01
Keep out of the reach and sight of children.

ONE Panadol OA Tablet contains paracetamol 1000 mg, the same amount of paracetamol as TWO standard paracetamol tablets.
Take 1 tablet as prescribed.
Peel back label and read instructions carefully before taking these tablets.
Do not exceed the stated dose
45mm
153mm
The manufacturer is GlaxoSmithKline Dungarvan Ltd., Co. Waterford, Ireland or Catalent UK Packaging Limited, Corby, Northamptonshire, NN18 8HS, U.K.
This information was prepared in February 2010


Packs of Panadol OA Tablets contain 100 tablets
The marketing authorisation holder is
GlaxoSmithKline Consumer Healthcare, Brentford, TW8 9GS, U.K. and all enquiries should be sent to this address.
3. How to take Panadol OA Tablets
^ Adults and children aged 12 years and over:
Take 1 tablet as prescribed.
Q • ONE Panadol OA Tablet contains paracetamol 1000 mg, the same amount as TWO standard paracetamol tablets.
• Do not take more frequently than every 4 hours.
• Do not take more than 4 tablets in 24 hours.
• Do not exceed the stated dose.
• Do not take with other paracetamol containing products.
• Do not give to children under 12 years. If you take too many tablets Immediate medical advice should be sought in the event of an overdose, even if you feel well because of the risk of delayed serious liver damage.
• If symptoms persist consult your doctor.

Like all medicines Panadol OA Tablets can have side effects, but not everybody gets them. A small number of people have had side effects. Stop taking the medicine and tell your doctor immediately if you experience:
• Allergic reactions which may be severe such as skin rash and itching sometimes with swelling of the mouth or face or shortness of breath
« Skin rash or peeling, or mouth ulcers
• Breathing problems. These are more likely if you have experienced them before when taking other painkillers such as ibuprofen and aspirin
• Unexplained bruising or bleeding
• Nausea, sudden weight loss, loss of appetite and yellowing of the eyes and skin.
If you do get any side effects, even those not mentioned in this information or if any of the side effects get serious, tell your doctor or pharmacist.
Panadol OA Tablets are white capsule-shaped tablets.
Active ingredient: Each tablet contains Paracetamol 1000 mg.
Other ingredients: Maize starch, pregelatinised starch, povidone, potassium sorbate (E 202), talc, stearic add, hypromellose and triacetin.
How to store Panadol OA Tablets Keep out of the reach and sight of children. Do not use this medicine after the 'EXP' date shown on the side of the pack
GlaxoSmithKline
ONE Panadol 0A Tablet contains paracetamol 1000 mg, the same amount of paracetamol as #TW0 !*ndard paracetamol tablets.
T*e1 tablet as prescribed.
•Peel back label and read instructions carefully before taking these tablets.
Do not exceed the stated dose CONTAINS PARACETAMOL. Do not take with any other paracetamol-containing products.
Immediate medical advice should be sought in the event of an overdose, even if you feel well.

466mm

466mm
Measurement Bar (mm)
0 5 10 15 20 25 30 35 TO 45 50 55 60 G5 70 75 80 85 80 95 108
|
(gsk/ GlaxoSmithKline |
SCHAWK! |
Project Name Brand Variant Component Country Drawing Nn. Dimensions |
|
Schawk Job No.: |
263154 | |
|
Version No.: |
02 |
Technical Spec Ref NPI Ref |
Panadol OA 1000mg Panadol
OA 1000mg 100 tablets
Label/Leaflet
UK
L655D001/02
45 x 153 mm & 45 x 466 mm

Master Colour Target Ref Factory Code Formulation Code SKU Code Copy Code
|
■ |
■ |
■ |
■ |
|
100% |
95% |
50% |
5% |
INCI Ref Code Pharma Code Barcode No. Mag & Bwr Printer Print Process No. of Colours Substrate Date
Brand Manager
N/A
N/A
N/A
N/A
N/A
N/A
N/A
N/A
N/A
N/A
N/A
MY Healthcare - Liverpool Flexo
1 (one) + Braille + Release Varnish 90gsm Black Label Gloss 10th February 2010 Kevin Grainger
|
Operator | |
|
QC |
3mm
3mm

GlaxoSmithKline
GSK Drawing No.: L655D001/02 Dimensions: 45 x 153 mm & 45x466 mm
Factory: Catalent
Component: Fix-A-Form Label
Date : 06th January 2009
Profile Keys:

Copy/Text Free Pharma Code Patch Release Varnish
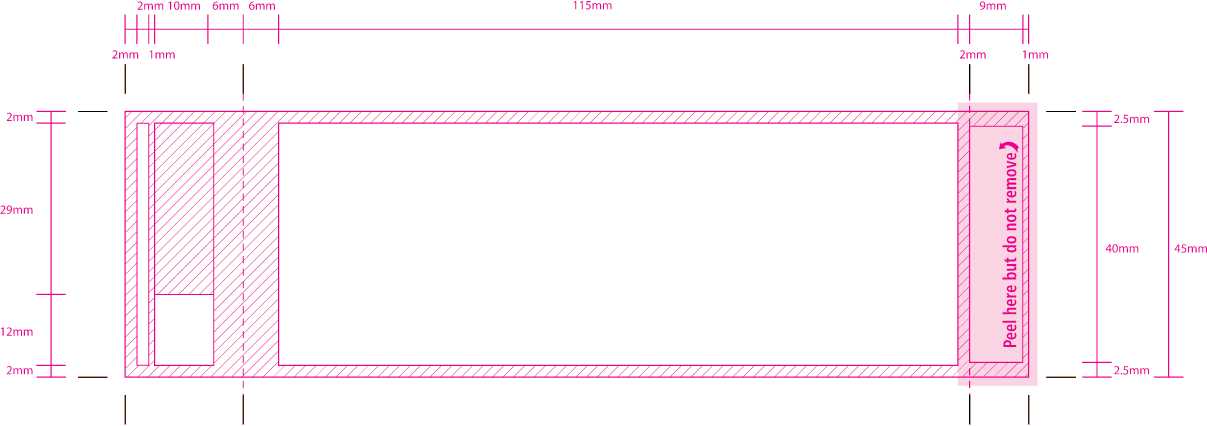
153mm
116mm
116mm
116mm
118mm

mm mo mm mo mm mo mo oo mo
mo oo om oo om om oo oo om mo oo mo oo oo mo mo oo mo
mo oo oo oo oo oo oo oo oo oo oo oo oo oo oo
oo mo om om om oo mm mm oo oo oo oo oo oo
om oo mm mm mm oo oo om oo oo oo oo oo oo
mo oo oo oo oo oo mo oo oo oo oo oo oo oo
oo oo oo oo oo oo oo oo oo oo oo oo oo oo
466mm

//// //// //// //// //// //// //// /OO //// //// //// //// //// /// //// ///^
¥ ¥ ¥
6mm 6mm 6mm
466mm
0 S 10 , 5 20 25 30 35 40 45 50 s5 GO 65 70 J5 80 85 00 95 100
Measurement Bar (mm)
|
(g |
SCHAWK! |
|
GlaxoSmithKline | |
|
Schawk Job No.: |
263154 |
|
Version No.: |
02 |
|
WL ■ ■■ 100% 95% 50% 5% |
r |
|
Project Name |
Panadol OA 1000mg |
|
Brand |
Panadol |
|
Variant |
OA 1000mg 100 tablets |
|
Component |
Label/Leaflet |
|
Country |
UK |
|
Drawing No. |
L655D001/02 |
|
Dimensions |
45 x 153 mm & 45 x 466 mm |
|
Technical Spec Ref |
N/A |
|
NPI Ref |
N/A |
|
Master Colour Target Ref |
N/A |
|
Factory Code |
N/A |
|
Formulation Code |
N/A |
|
SKU Code |
N/A |
|
Copy Code |
N/A |
|
INCI Ref Code |
N/A |
|
Pharma Code |
N/A |
|
Barcode No. |
N/A |
|
Mag & Bwr |
N/A |
|
Printer |
MY Healthcare - Liverpool |
|
Print Process |
Flexo |
|
No. of Colours |
1 (one) + Braille + Release Varnish |
|
Substrate |
90gsm Black Label Gloss |
|
Date |
10th February 2010 |
|
Brand Manager |
Kevin Grainger |
BRAILLE EXPLANATIONS AND TRANSLATIONS
|
Operator | |
|
QC |
3mm
3mm

GlaxoSmithKline
GSK Drawing No.: L655D001/02 Dimensions: 45 x 153 mm & 45x466 mm
Factory: Catalent
Component: Fix-A-Form Label
Date : 06th January 2009
Profile Keys:

Copy/Text Free Pharma Code Patch Release Varnish
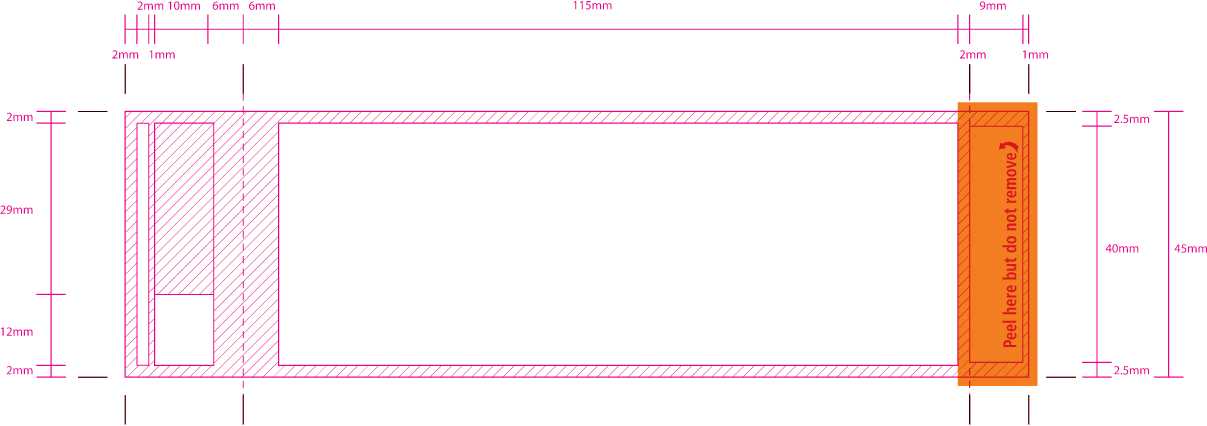
153mm
116mm
116mm
116mm
118mm
466mm

6mm 6mm 6mm
466mm
0 S 10 , 5 20 25 30 35 40 45 50 s5 GO 65 70 J5 80 85 00 95 100
Measurement Bar (mm)

SCHAWK!
|
(g |
SCHAWK! | |
|
GlaxoSmithKline | ||
|
Schawk Job No.: |
263154 | |
|
Version No.: |
02 | |
|
□e 1 S 5% |
© | |
|
Project Name |
Panadol OA 1000mg |
|
Brand |
Panadol |
|
Variant |
OA lOOOmg 100 tablets |
|
Component |
Label/Leaflet |
|
Country |
UK |
|
Drawing No. |
L655D001/02 |
|
Dimensions |
45 x 153 mm & 45 x 466 mm |
|
Technical Spec Ref |
N/A |
|
NPI Ref |
N/A |
|
Master Colour Target Ref |
N/A |
|
Factory Code |
N/A |
|
Formulation Code |
N/A |
|
SKU Code |
N/A |
|
Copy Code |
N/A |
|
INCI Ref Code |
N/A |
|
Pharma Code |
N/A |
|
Barcode No. |
N/A |
|
Mag & Bwr |
N/A |
|
Printer |
MY Healthcare - Liverpool |
|
Print Process |
Flexo |
|
No. of Colours |
1 (one) + Braille + Release Varnish |
|
Substrate |
90gsm Black Label Gloss |
|
Date |
10th February 2010 |
|
Brand Manager |
Kevin Grainger |
|
Operator | |
|
QC |
MHRA
Schawk, Kingsway North, Newcastle, Tyne & Wear NE11 0IH, UK. T +44 (0) 191 491 7777 F +44 (0) 191 487 6673 V2 06Nov2008