Panadol Actifast
>1
FRONT

GSK Drawing No.: D328D033/05
Dimensions:
Factory:
Component:
Date:
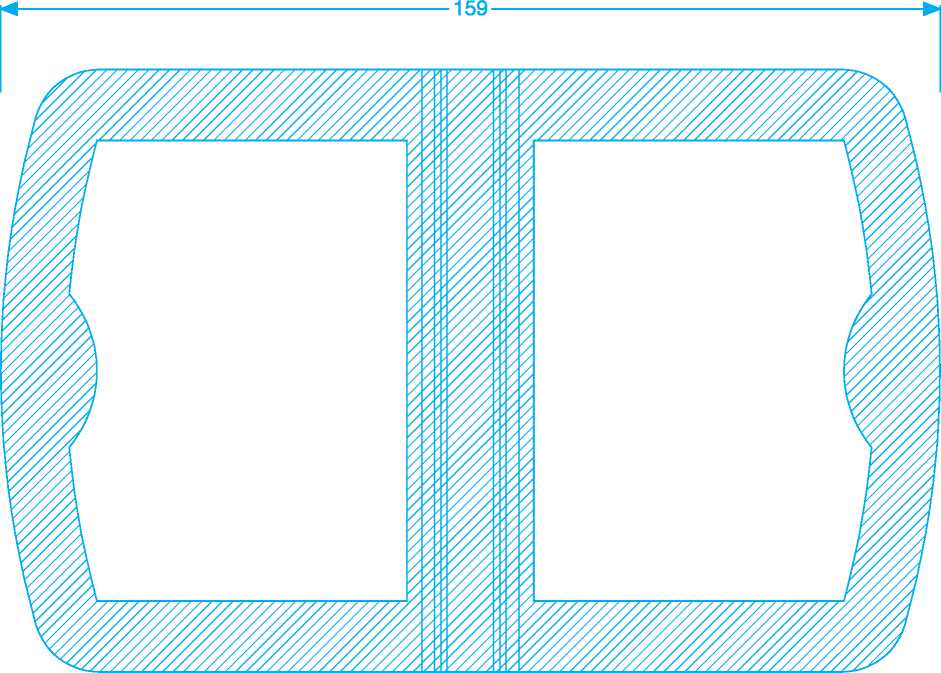
102 x 159 mm Dungarvan Skins carton outer & inner 18th April 2011
Profile Keys:
Varnish Free Copy/Text Free Print Free Dot Matrix Code

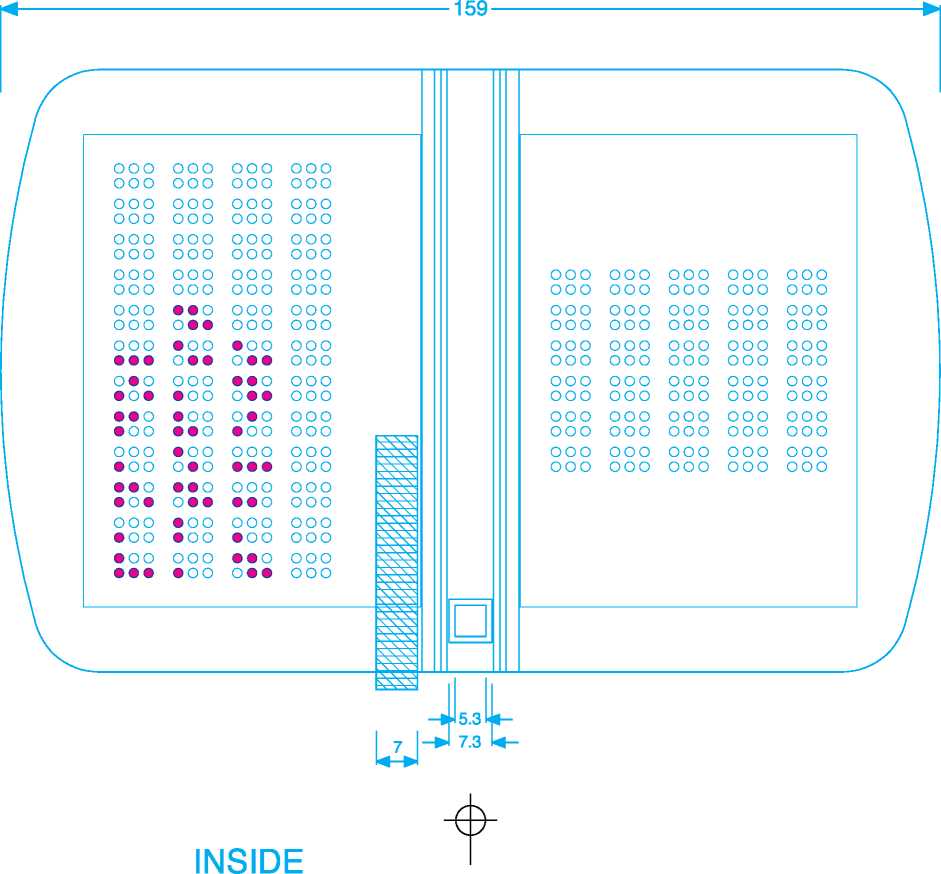
159
it
Please read right through the information on the outside and inside of this pack before you start using this medicine.
If you have any questions, or if there is anything you do not understand, ask your pharmacist.
Panadol ActiFast has a unique formulation which gets to the source of pain fast It acts faster than standard paracetamol tablets you can swallow to give fast pain relief of headaches, including migraine and tension headaches, toothache, backache, rheumatic and muscle pain and period pain. It also relieves sore throat and the fever, aches and pains of colds and flu.
Contains paracetamol. Do not take with any other paracetamol-containing products.
40
Panadol ActiFast has a unique formulation which gets to the source of pain fast.
The active Ingredient Is paracetamol which Is a painkiller and also reduces your temperature when you have a fever.
□ Do not take Panadol ActiFast:
• If you have ever had an allergic reaction to
paracetamol or to any of the other Ingredients (listed In Section 4).
OAsk your doctor before you take this medicine: • if you are on a controlled sodium diet. Each 2 tablet dose contains 346 mg of sodium.
O If you are taking other medicines
Talk to your doctor or pharmacist before taking these tablets If you are taking any prescribed /V medicines; particularly metodopramlde or domperldone //. (for nausea [feeling sick] or vomiting [being sick]) or colestyramlne (to lower blood cholesterol) if you take blood thinning drugs (anticoagulants e.g. warfarin) and you need to take a pain reliever on a dally basis, talk to your doctor because of the risk of bleeding. But you can still take occasional doses of Panadol ActiFast at the same time as anticoagulants.
O Pregnancy and breast feeding
Talk to your doctor before taking Panadol ActiFast if you are pregnant. You can take this product whilst breast feeding.
a
V
Immediate medical advice should be sought in the event of an overdose, even if you feel well, because of the risk of delayed, serious liver damage.
If your symptoms persist consult your doctor.
Each tablet contains Paracetamol 500 mg. Also contains potassium sorbate (E 202). Do not store above 25°C.
Keep out of the reach and sight of children.
GlaxoSmithKline Consumer Healthcare, Brentford, TW8 9GS, U.K.
GlaxoSmithKline PL 00071/0444 40U572F D328125/09

Tablets
Paracetamol
Tension headache V Toothache •/ Period pain </ Easy on the stomach V
Like all medicines, Panadol ActiFast can have side effects but not everybody gets them. A small number of people have had side effects. Stop taking the medicine and tell

your doctor Immediately If you experience:
• Allergic reactions which may be severe such as sldn rash and itching sometimes with swelling of the mouth or face or shortness of breath
• Skin rash or peeling, or mouth ulcers
• Breathing problems. These are more likely if you have experienced them before when taking other painkillers such as Ibuprofen and aspirin
• Unexplained bruising or bleeding
• Nausea, sudden weight loss, loss of appetite and yellowing of the eyes and skin.
If you do get any side effects, even those not mentioned in this Information, tell your doctor or pharmacist.
If your symptoms continue or your headache becomes persistent, see your doctor.
Active ingredient: Each tablet contains Paracetamol 500 mg. Other Ingredients: Sodium bicarbonate, starch pregelatinised, povidone, maize starch, potassium sorbate (E 202), microcrystalline cellulose, magnesium stearate, carnauba wax, titanium dioxide (E 171), polydextrose, hypromellose, glycerol triacetate and polyethylene glycol.
Packs of Panadol ActiFast contain 8 or 14 tablets.
The marketing authorisation holder Is GlaxoSmithKline Consumer Healthcare, Brentford, TW8 9GS, U.K. and all enquiries should be sent to this address.
The manufacturer is GlaxoSmithKline Dungarvan Ltd.,
Co. Waterford, Ireland.
This Information was last revised In July 2010.
Panadol, ActiFast and the Triangle device are registered trade marks of the GlaxoSmithKline group of companies.
40U572F D328125/09
0 5 10 15 20 25 30 35 45 50 55 60 55 70 75 80
IiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIi
90
s.s Measurement Bar lllllll (mm)
|
GlaxoSmithKline |
SCHAWK! |
Project Name Brand Variant Component Country Drawing No. Dimensions |
|
Schawk Job No.: |
300081 | |
|
Version No.: |
02 |
Technical Spec Ref NPI Ref |
Dungarvan profile change
Panadol
Actifast
14 Compak
UK
D328D033/05 102x159 mm

P.368
Green
P.485
Blue
P.347
Green
P.2765
Blue
Factory Code 40U572F
Copy Code D328125/09

Process Process
Cyan Black
100% 95% 50% 5%
■ala
INCI Ref Code DataMatrix Code Barcode No.
Mag t Bwr Printer Print Process No. of Colours Substrate Date
Brand Manager
4276
5000347087141 Mag 80% Bwr 30 Chesapeake Belfast Litho / Offset 6 (Six) + Braille + Varnish FBB
31st May 2011 Hannah Norbury
JOB STATUS: ARTWORK
4276
|
Operator |
4 | |
|
ac |
Page 1 of |

GSK Drawing No.: D328D033/05
Dimensions:
Factory:
Component:
Date:
102 x 159 mm Dungarvan Skins carton outer & inner 18th April 2011
Profile Keys:
Varnish Free Copy/Text Free Print Free Dot Matrix Code

Panadol ActiFast has a unique formulation which gets to the source of pain fast.
The active Ingredient Is paracetamol which Is a painkiller and also reduces your temperature when you have a fever.
□ Do not take Panadol ActiFast:
• If you have ever had an allergic reaction to
paracetamol or to any of the other Ingredients (listed In Section 4).
OAsk your doctor before you take this medicine: • if you are on a controlled sodium diet. Each 2 tablet dose contains 346 mg of sodium.
O If you are taking other medicines
Talk to your doctor or pharmacist before taking these tablets If you are taking any prescribed /V medicines; particularly metodopramlde or domperldone /a (for nausea [feeling sick] or vomiting [being sick]) or colestyramlne (to lower blood cholesterol) if you take blood thinning drugs (anticoagulants e.g. warfarin) and you need to take a pain reliever on a dally basis, talk to your doctor because of the risk of bleeding. But you can still take occasional doses of Panadol ActiFast at the same time as anticoagulants.
O Pregnancy and breast feeding
Talk to your doctor before taking Panadol ActiFast if you are pregnant. You can take this product whilst breast feeding.
it
Please read right through the information on the outside and inside of this pack before you start using this medicine.
If you have any questions, or if there is anything you do not understand, ask your pharmacist.
Panadol ActiFast has a unique formulation which gets to the source of pain fast It acts faster than standard paracetamol tablets you can swallow to give fast pain relief of headaches, including migraine and tension headaches, toothache, backache, rheumatic and muscle pain and period pain. It also relieves sore throat and the fever, aches and pains of colds and flu.
Contains paracetamol. Do not take with any other paracetamol-containing products.
IB
a
V
Immediate medical advice should I"1 sought in the event of an overdose, even if JjsSJSS i? you feel well, becausn of the risk of deNed, serio1 's l'uer damage.
tf your symptoms pei st snsultys '<’ rtor.
Each tablet contains Paracetamol _,00 mg. Also contains potassiun orbate(E2C ). Donotstc above 25°C.
Keep out of the r*>ach a"d sight of children.
GlaxoSmithKline Consumer Healthcare, Brentford, TW8 9GS, U.K.
GlaxoSmithKline PL 00071/0444

Tablets
Paracetamol
Tension headache A Toothache •/ Period pain </ Easy on the stomach V
Like all medicines, Panadol ActiFast can have side effects but not everybody gets them. A small number of people have had side effects. Stop taking the medicine and tell

your doctor Immediately If you experience:
• Allergic reactions which may be severe such as sldn rash and itching sometimes with swelling of the mouth or face or shortness of breath
• Skin rash or peeling, or mouth ulcers
• Breathing problems. These are more likely if you have experienced them before when taking other painkillers such as Ibuprofen and aspirin
• Unexplained bruising or bleeding
• Nausea, sudden weight loss, loss of appetite and yellowing of the eyes and skin.
If you do get any side effects, even those not mentioned in this Information, tell your doctor or pharmacist.
If your symptoms continue or your headache becomes persistent, see your doctor.
Active ingredient: Each tablet contains Paracetamol 500 mg. Other Ingredients: Sodium bicarbonate, starch pregelatinised, povidone, maize starch, potassium sorbate (E 202), microcrystalline cellulose, magnesium stearate, carnauba wax, titanium dioxide (E 171), polydextrose, hypromellose, glycerol triacetate and polyethylene glycol.
Packs of Panadol ActiFast contain 8 or 14 tablets.
The marketing authorisation holder Is GlaxoSmithKline Consumer Flealthcare, Brentford, TW8 9GS, U.K. and all enquiries should be sent to this address.
The manufacturer is GlaxoSmithKline Dungarvan Ltd.,
Co. Waterford, Ireland.
This information was last revised In July 2010.
Panadol, ActiFast and the Triangle device are registered trade marks of the GlaxoSmithKline group of companies.
40U572F D328125/09
0 5 10 15 20 25 30 35 40 45 50 55 60 55 70 75 80
IiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIiiiiIi
90
s.s Measurement Bar lllllll (mm)
BRAILLE EXPLANATIONS AND TRANSLATIONS
|
GlaxoSmithKline |
SCHAWK! |
Project Name Brand Variant Component Country Drawing No. Dimensions |
|
Schawk Job No.: |
300081 | |
|
Version No.: |
02 |
Technical Spec Ref NPI Ref |
Dungarvan profile change
Panadol
Actifast
14 Compak
UK
D328D033/05 102x159 mm

P.368 P.485 P.347 P.2765
Green Blue Green Blue

Process Process
Cyan Black
100% 95% 50% 5%
■ala
Factory Code 40U572F
Copy Code D328125/09
INCI Ref Code DataMatrix Code Barcode No.
Mag t Bwr Printer Print Process No. of Colours Substrate Date
Brand Manager
4276
5000347087141 Mag 80% Bwr 30 Chesapeake Belfast Litho / Offset 6 (Six) + Braille + Varnish FBB
31st May 2011 Hannah Norbury
|
• |
• | |||||
|
• |
• |
• |
• |
• | ||
|
• |
• |
• |
• | |||
|
P |
A |
N |
A D |
0 |
L | |
|
• |
• • |
• |
• •• |
• |
• |
• |
|
• • |
• • |
• |
• • | |||
|
• |
• |
• | ||||
|
A |
C |
T |
1 F |
A |
S |
T |
|
• |
• |
• |
• • |
• |
• | |
|
• • |
• |
• • |
• • |
• | ||
|
• |
• |
• |
• | |||
|
T |
A |
1 |
L E |
T |
s |
Panadol
Actifast
Tablets
4276
|
Operator |
4 | |
|
ac |
Page 2 of |

GSK Drawing No.: D328D033/05
Dimensions:
Factory:
Component:
Date:
102 x 159 mm Dungarvan Skins carton outer & inner 18th April 2011
Profile Keys:
Varnish Free Copy/Text Free Print Free Dot Matrix Code

it
V

90
s.s Measurement Bar lllllll (mm)
BRAILLE EXPLANATIONS AND TRANSLATIONS
|
GlaxoSmithKline |
SCH/UVK! |
Project Name Brand Variant Component Country Brewing No. Bimensions |
|
Schawk Job No.: |
300081 | |
|
Version No.: |
02 |
Technical Spec Ref NPI Ref |
P.485
Blue
Dungarvan profile change
Panadol
Actifast
14 Compak
UK
D328D033/05 102x159 mm

P.368
Green
P.347
Green
P.2765
Blue
Factory Code 40U572F
Copy Code D328125/09
|
• |
• | |||||
|
• |
• |
• |
• |
• | ||
|
• |
• |
• |
• | |||
|
P |
it |
N |
A D |
0 |
L | |
|
• |
• • |
• |
• •• |
• |
• |
• |
|
• • |
• • |
• |
• • | |||
|
• |
• |
• | ||||
|
A |
C |
T |
1 F |
A |
S |
T |
|
• |
• |
• |
• • |
• |
• | |
|
• • |
• |
• • |
• • |
• | ||
|
• |
• |
• |
• | |||
|
T |
A |
1 |
L E |
T |
s |
Panadol
Actifast
Tablets

Process Process
Cyan Black

100% 95% 50% 5%
Bala
INCI Ref Code DataMatrix Code Barcode No.
Mag t Bwr Printer Print Process No. of Colours Substrate Bate
Brand Manager
4276
5000347087141 Mag 80% Bwr 30 Chesapeake Belfast Litho / Offset 6 (Six) + Braille + Varnish FBB
31st May 2011 Hannah Norbury
|
Operator |
4 | |
|
ac |
Page 3 of |
4276
ADVANCEMENT DIRECTION
->
Pharma code Ref. No. 1197
Pharma code Ref. No. 1197
Pharma code Ref. No. 1197
Pharma code Ref. No. 1197


GSK Market is responsible for this product, its design and content.
Ensure the artwork is thoroughly checked, all the text proof-read and approved.
RSC GSK is responsible for site technical requirements and pre-press suitability.
TEXT SIZE CONTAINED IN THIS ARTWORK
Body text size: 6.0pt Smallest text size: 6.0pt Microtext: N
Artwork copyright is the property of the GlaxoSmithKline Group of Companies
All suppliers providing a service to GSK for printed components of any description must ensure that they have a licence for all fonts / software used in conjunction with GSK artwork. The distribution and use of fonts / software without a licence constitutes an intellectual property infringement. GSK will not accept any liability for the breach of third party intellectual property rights by printed component suppliers. The GSK certification / audit process requires suppliers to declare that they do not use unlicensed fonts / software and may require the supplier to produce evidence of such licence to GSK.
ATTENTION • ATTENTION • ATTENTION • ATTENTION • ATTENTION • ATTENTION • ATTENTION • ATTENTION
To Ensure Accurate PDF Viewing and Printing:
FOR SCREEN VIEWING: Use Adobe Acrobat 7 Professional or Adobe Acrobat Reader, Standard or Professional (higher than 7).
Overprint Preview must be activated for accurate on screen viewing.
FOR PRINTING: Use only Acrobat Professional version 7 or higher. "Apply Overprint Preview" or "Simulate Overprinting" must be activated in the print settings for printing accurate hard copies.
180 mm Measuring Bar
If a status identification banner DOES NOT appear on this document, THEN this document has NOT been printed from the Global Pack Management system.
GSK Market
is responsible to advise RSC in case changes required impact the followings:
Formulation Tablet embossing Storage conditions Shelf Life
|
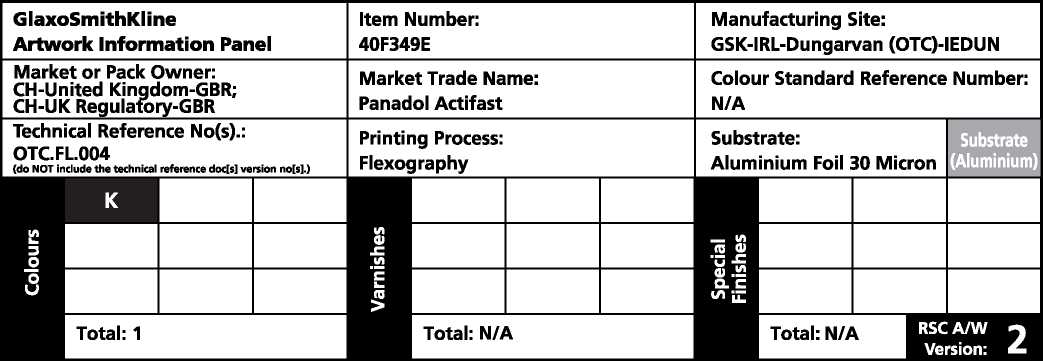
Dungarvan - Additional Artwork Information Panel | |
|
Pharmacode No. |
1197 |
|
Varnish Type |
N/A |
|
Generic Specification Reference No. |
OTC.FL.SPEC.006 |
Page 1 of 1