Penthrox 3 Ml Inhalation Vapour Liquid
SUMMARY OF PRODUCT CHARACTERISTICS
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
1 NAME OF THE MEDICINAL PRODUCT
PENTHROX 3mL inhalation vapour, liquid
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 3 mL of methoxyflurane 99.9%.
For full list of excipients see, section 6.1.
3 PHARMACEUTICAL FORM
Inhalation vapour, liquid.
Clear, almost colourless, volatile liquid, with a characteristic fruity odour.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Emergency relief of moderate to severe pain in conscious adult patients with trauma and associated pain. See sections 4.2 and 5.1.
4.2 Posology and method of administration
PENTHROX should be self-administered under supervision of a person trained in its administration, using the hand held PENTHROX Inhaler.
Adults
One bottle of 3 mL PENTHROX to be vaporised in a PENTHROX inhaler. On finishing the 3 mL dose, another 3 mL may be used. Dose of PENTHROX should not exceed 6 mL in a single administration. Methoxyflurane may cause renal failure if the recommended dose is exceeded. The lowest effective dosage of PENTHROX to provide analgesia should be used.
Onset of pain relief is rapid and occurs after 6 - 10 inhalations. Patients should be instructed to inhale intermittently to achieve adequate analgesia. Patients are able to assess their own level of pain and titrate the amount of PENTHROX inhaled for adequate pain control. Continuous inhalation provides analgesic relief for up to 2530 minutes. Intermittent inhalation may provide longer analgesic relief. Patients should be advised to take the lowest possible dose to achieve pain relief.
The frequency at which PENTHROX can be safely used is not established. Administration on consecutive days is not recommended and the total dose to a patient in a week should not exceed 15 ml (see section 4.4).
Children
PENTHROX should not be used in children under 18 years. Method of Administration
Instructions on the preparation of the PENTHROX Inhaler and correct administration are provided in the Figures below.
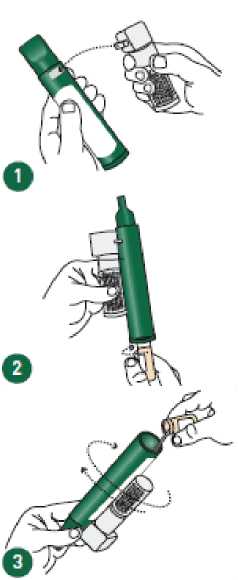
Ensure the Activated Carbon (AC) Chamber is 1 inserted into the dilutor hole on the top of the PENTHROX Inhaler.
Remove the cap of the bottle by hand. Alternatively, use the base of the PENTHROX 2 Inhaler to loosen the cap with a 'A turn. Separate the Inhaler from the bottle and remove the cap by hand.
Tilt the PENTHROX Inhaler to a 45° angle and pour the total contents of one PENTHROX bottle into the base of the Inhaler whilst rotating.
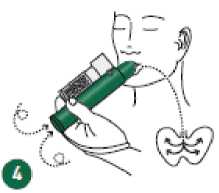
Place wrist loop over patient’s wrist. Patient inhales through the mouthpiece of the 4 PENTHROX Inhaler to obtain analgesia. First few breaths should be gentle and then breathe normally through Inhaler.
Patient exhales into the PENTHROX Inhaler. The exhaled vapour passes through the AC Chamber to adsorb any exhaled methoxyflurane.
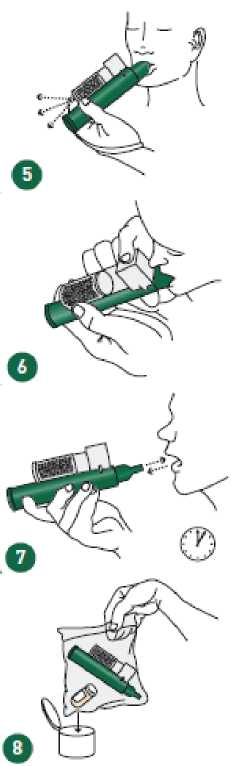
6
7
8
If stronger analgesia is required, patient can cover dilutor hole on the AC chamber with finger during use.
Patient should be instructed to inhale intermittently to achieve adequate analgesia. Continuous inhalation will reduce duration of use. Minimum dose to achieve analgesia should be administered.
Replace cap onto PENTHROX bottle. Place used PENTHROX Inhaler and used bottle in sealed plastic bag and dispose of responsibly.
Healthcare professional administering PENTHROX must provide and explain the Product Information Leaflet to the patient.
4.3 Contraindications
Use as an anaesthetic agent.
Hypersensitivity to PENTHROX or any fluorinated anaesthetic.
Malignant hyperthermia: patients with known or genetically susceptible to malignant hyperthermia or a history of severe adverse reactions in either patient or relatives.
Patients who have a history of showing signs of liver damage after previous methoxyflurane use or halogenated hydrocarbon anaesthesia.
Clinically significant renal impairment.
Altered level of consciousness due to any cause including head injury, drugs, or alcohol.
Clinically evident cardiovascular instability. Clinically evident respiratory depression.
4.4 Special warnings and precautions for use
Renal disease
Methoxyflurane causes significant nephrotoxicity at high doses. Nephrotoxicity is also related to the rate of metabolism. Therefore factors that increase the rate of metabolism such as drugs that induce hepatic enzymes can increase the risk of toxicity with methoxyflurane as well as sub-groups of people with genetic variations that may result in fast metaboliser status (see section 4.5). Nephrotoxicity is thought to be associated with inorganic fluoride ions, a metabolic break-down product. Toxicity in the past when used as an anaesthetic agent has been determined to be associated with serum levels greater than 40 pmol/L. Following a single dose of 3 mL serum levels did not exceed 10 pmol/L.
Despite this safety margin the lowest effective dose of methoxyflurane should be administered, especially in the elderly or patients with other known risk factors of renal disease. In addition, methoxyflurane should be cautiously used in patients diagnosed with clinical conditions that would pre-dispose to renal injury.
Liver disease
Methoxyflurane is metabolised in the liver, therefore increased exposures in patients with hepatic impairment can cause toxicity. PENTHROX must not be used in patients who have a history of showing signs of liver damage after previous methoxyflurane use or halogenated hydrocarbon anaesthesia. PENTHROX should be used with care in patients with underlying hepatic conditions or with risks for hepatic dysfunction (such as enzyme inducers - see also section 4.5).
It has been reported that previous exposure to halogenated hydrocarbon anaesthetics (including methoxyflurane when used in the past as an anaesthetic agent), especially if the interval is less than 3 months, may increase the potential for hepatic injury.
Cautious clinical judgement should be exercised when PENTHROX is to be used more frequently than on one occasion every 3 months.
Cardiovascular system depression / Use in elderly
Potential effects on blood pressure and heart rate are known class-effects of high dose methoxyflurane used in anaesthesia and other anaesthetics. They do not appear to be significant at the analgesic doses. There is no particular pattern to the patients’ systolic BP levels after methoxyflurane administration as an analgesic across age groups. However, as the risk may potentially be increased for older people with hypotension and bradycardia, caution should be exercised in the elderly due to possible reduction in blood pressure.
Central nervous system effects
Secondary pharmacodynamic effects including potential CNS effects such as sedation, euphoria, amnesia, ability to concentrate, altered sensorimotor coordination and change in mood are also known class-effects. Selfadministration of methoxyflurane in analgesic doses will be limited by occurrence of CNS effects, such as sedation.
Additionally, the CNS effects can be a risk factor for potential abuse. Occupational exposure
Healthcare professionals who are regularly exposed to patients using PENTHROX inhalers should be aware of any relevant occupational health and safety guidelines for the use of inhalational agents. To reduce occupational exposure to methoxyflurane, the PENTHROX Inhaler should always be used with the Activated Carbon (AC) Chamber which adsorbs exhaled methoxyflurane. Multiple use of PENTHROX Inhaler without the AC Chamber creates additional risk. Elevation of liver enzymes, blood urea nitrogen and serum uric acid have been reported in exposed maternity ward staff in delivery wards when methoxyflurane was used in the past in obstetric patients at the time of labour and delivery.
Frequent repeated use
Due to the limitations on the dose of PENTHROX (maximum - 6ml) and the duration of pain relief, PENTHROX is not appropriate for providing relief of break-through pain/exacerbations in chronic pain conditions. PENTHROX is also not appropriate for relief of trauma related pain in closely repeated episodes for the same patient.
Butylated hydroxytoluene
PENTHROX contains the excipient, butylated hydroxytoluene (E321), a stabiliser. Butylated hydroxytoluene may cause local skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes. See section 6.1.
4.5 Interaction with other medicinal products and other forms of interaction
The metabolism of methoxyflurane is mediated by the CYP 450 enzymes particularly CYP 2E1 and to some extent CYP 2A6. It is possible that enzyme inducers (such as alcohol or isoniazid for CYP 2E1 and phenobarbital or rifampicin for CYP 2A6) which increase the rate of methoxyflurane metabolism might increase its potential toxicity and they should be avoided concomitantly with methoxyflurane.
Concomitant use of PENTHROX with CNS depressants, such as opioids, sedatives or hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating antihistamines and alcohol may produce additive depressant effects. If opioids are given concomitantly with PENTHROX, the patient should be observed closely, as is normal clinical practice with opioids.
Concomitant use of methoxyflurane with medicines (e.g. contrast agents and some antibiotics) which are known to have a nephrotoxic effect should be avoided as there may be an additive effect on nephrotoxicity. Antibiotics with known nephrotoxic potential include tetracycline, gentamicin, colistin, polymyxin B and amphotericin B. It is advisable to avoid using sevoflurane anaesthesia following methoxyflurane analgesia, as sevoflurane increases serum fluoride levels and nephrotoxicity of methoxyflurane is associated with raised serum fluoride.
There are no reported drug interactions when used at the analgesic dosage (3 - 6 mL).
When methoxyflurane was used for anaesthesia at the higher doses of 40 - 60 mL, there were reports of:
a) Drug interaction with hepatic enzyme inducers (eg barbiturates) increasing metabolism of methoxyflurane and resulting in a few reported cases of nephrotoxicity. There is insufficient information to show whether enzyme induction affects liver damage after an analgesic dose of methoxyflurane.
b) Reduction of renal blood flow and hence anticipated enhanced renal effect when used in combination with drugs (eg barbiturates) reducing cardiac output.
c) Class effect on cardiac depression which may be enhanced by other cardiac depressant drugs, eg intravenous practolol during cardiac surgery.
4.6 Fertility, pregnancy and lactation
Fertility
No clinical data on effects of methoxyflurane on fertility are available. Limited data from animal studies do no indicate any effects on sperm morphology.
Pregnancy
Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
Where methoxyflurane has been used for obstetric analgesia in pregnant women, there has been a single report of neonatal respiratory depression associated with a high fetal level of methoxyflurane. However, when low concentrations were administered, or the duration of higher concentrations was kept short, per recommended posology, methoxyflurane was found to have little effect on the fetus. No fetal complications were reported to result from methoxyflurane analgesia in the mother in all the studies completed in obstetric analgesia.
As with all medicines care should be exercised when administered during pregnancy especially the first trimester.
Breast-feeding
There is insufficient information on the excretion of methoxyflurane in human milk._Caution should be exercised when methoxyflurane is administered to a nursing mother.
4.7 Effects on ability to drive and use machines
Methoxyflurane may have a minor influence on the ability to drive and use machines. Dizziness, somnolence and drowsiness may occur following the administration of methoxyflurane (see section 4.8). Patients should be advised not to drive or operate machinery if they are feeling drowsy or dizzy.
4.8 Undesirable effects
Summary of safety profile
The most common non-serious reactions are CNS type reactions such as dizziness, and somnolence (>1/100 to <1/10), and are generally easily reversible.
Serious dose-related nephrotoxicity has only been associated with methoxyflurane when used in large doses over prolonged periods during general anaesthesia. Methoxyflurane is therefore no longer used for anaesthesia. See section 4.4 under renal disease. The recommended maximum dose for PENTHROX should therefore not be exceeded.
Tabulated list of adverse reactions
The adverse drug reactions observed in PENTHROX clinical studies in analgesia are listed in the table below, classified according to frequency (common >1/100 to <1/10; uncommon >1/1,000 to <1/100).
|
MedDRA System Organ Class |
Common >1/100 to <1/10 |
Uncommon >1/1,000 to <1/100 |
|
Nervous system disorders |
Amnesia Anxiety Depression Dizziness Dysarthria Dysgeusia Euphoria Headache Sensory neuropathy Somnolence |
Paraesthesia |
|
Cardiac disorders |
Hypotension | |
|
Eye disorders |
Diplopia | |
|
Respiratory, thoracic and mediastinal disorders |
Coughing | |
|
Gastrointestinal disorders |
Dry mouth Nausea |
Oral discomfort |
|
General disorders |
Feeling drunk |
Fatigue Feeling abnormal Increased appetite Shivering |
|
Skin and subcutaneous tissue disorders |
Sweating |
Post-marketing experience
Rare (>1/10,000 to <1/1,000) reports of hepatic failure/hepatitis have been observed with analgesic use of methoxyflurane.
Other events linked to the methoxyflurane use in analgesia (in addition to the reactions from clinical trials listed above), including reports from the literature include:
- Nervous system disorders: drowsiness, agitation, restlessness, dissociation, affect lability, disorientation, altered state of consciousness
- Respiratory system: choking, hypoxia, oxygen saturation decreased
- Cardiovascular system: blood pressure fluctuation
- Gastrointestinal: vomiting
- Hepatic: hepatitis, increased liver enzymes, jaundice, liver injury
- Renal: increased serum uric acid, urea nitrogen and creatinine, renal failure
- Eyes: blurred vision, nystagmus Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Patients should be observed for signs of drowsiness, pallor and muscle relaxation following methoxyflurane administration.
High doses of methoxyflurane cause dose related nephrotoxicity. High output renal failure has occurred several hours or days after the administration of repeated high analgesic or anaesthetic doses of methoxyflurane.
5.1 Pharmacodynamic properties
Pharmacodynamic effects
Methoxyflurane vapour provides analgesia when inhaled at low concentrations. After methoxyflurane administration, drowsiness may occur. During methoxyflurane administration, the cardiac rhythm is usually regular. The myocardium is only minimally sensitised to adrenaline by methoxyflurane. At analgesic therapeutic doses pain relief may lead to some decrease in blood pressure. This may be accompanied by bradycardia.
Clinical efficacy and safety
The efficacy and safety of PENTHROX was demonstrated in MEOF-001, a randomised, double-blind, multi-centre, placebo controlled study in the treatment of acute pain in patients with minor trauma presenting to an Emergency Department. 300 patients were recruited (149 received methoxyflurane and 149 received placebo in a 1:1 ratio). Patients with a pain score of > 4 to < 7 on the Numerical Rating Scale were eligible for the study. The mean pain scores (Visual Analogue Scale) observed at baseline were similar in the methoxyflurane (64.8) and placebo (64.0) groups. The primary efficacy variable, the estimated mean change in VAS pain from Baseline to 5 min, 10 min, 15 min and 20 min, was greater for the methoxyflurane group (23.1, -28.9, -34.0 and -35.0 respectively) when compared to the placebo group (11.3, -14.8, -15.5 and -19.0 respectively). Overall, there was a highly significant difference between the methoxyflurane and placebo group (estimated treatment effect -15.1; 95% CI -19.2 to -11.0; p<0.0001). The greatest treatment effect was seen at 15 minutes (estimated treatment effect of -18.5). An analysis was undertaken where a responder was defined as a patient who experienced at least a 30% improvement from baseline VAS pain score. Results of this analysis indicated that percentage of responders at 5, 10, 15 and 20 mins was significantly greater for the methoxyflurane group (51.0%, 57.7%, 63.8%, 63.8%) when compared to the placebo group (23.5%, 30.9%, 33.6%, 37.6%), with p < 0.0001 at each time-point. A total of 126 patients (84.6%) in the methoxyflurane group experienced their first pain relief after 1-10 inhalations in comparison to 76 patients (51%) in the placebo group.
5.2 Pharmacokinetic properties
Absorption
Methoxyflurane has the following partition coefficients:
• a water/gas coefficient of 4.5,
• a blood/gas coefficient of 13 and
• an oil/gas coefficient of 825
Methoxyflurane enters the lungs in the form of a vapour and is rapidly transported into the blood, therefore there is a rapid onset of analgesic action.
Distribution
Methoxyflurane has a high oil/gas coefficient hence methoxyflurane is highly lipophilic. Methoxyflurane has great propensity to diffuse into fatty tissues where it forms a reservoir from which it is released slowly over days.
Metabolism
Biotransformation of methoxyflurane occurs in man. Methoxyflurane is metabolised by dechlorination and o-demethylation in the liver, mediated by CYP 450 enzymes particularly CYP 2E1 and CYP 2A6. Methoxyflurane is metabolised to free fluoride, oxalic acid, difluoromethoxyacetic acid, and dichloroacetic acid. Both free fluoride and oxalic acid can cause renal damage at concentrations higher than those achievable with single analgesic dose use. Methoxyflurane is more susceptible to metabolism than other halogenated methyl ethyl ethers and has greater propensity to diffuse into fatty tissues. Hence methoxyflurane is released slowly from this reservoir and becomes available for biotransformation for many days.
Excretion
Approximately 60% of methoxyflurane uptake is excreted in the urine as organic fluorine, fluoride and oxalic acid; the remainder is exhaled unaltered or as carbon dioxide. Higher peak blood fluoride levels may be obtained earlier in obese than in non-obese people, and in the elderly.
5.3 Preclinical safety data
Embryo-fetal development
In studies in mice and rats, methoxyflurane crossed the placenta but demonstrated no evidence of embryotoxic or teratogenic properties. However, delayed fetal development (reduced fetal body weight and decreased ossification) was observed following repeated dosing over 9 days. The no observed adverse effect level (NOAEL) for embryo-fetal development was 0.006% - 4h/day in mice and close to 0.01% - 8 h/day in rats. The NOAELs in mouse and rat represent a 1- to 2-fold margin on a mg/kg basis and a 0.1- to 0.3-fold margin on a mg/m2 basis versus the proposed maximum clinical dose. As PENTHROX is not intended for daily use, the risk of delayed fetal development is considered to be very low.
Hepatic effects
Repeated intermittent or continuous administration of subanaesthetic concentrations of methoxyflurane has been associated with limited and commonly reversible hepatic changes (fatty metamorphosis, elevated ALT/AST) in several species. A NOAEL has not been established. These effects were seen at exposures considered sufficiently in excess of those anticipated through normal clinical use of the product.
6.1 List of excipients
Butylated hydroxytoluene E321 (stabiliser).
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
36 months.
6.4 Special precautions for storage
This medicinal product does not require any special temperature storage conditions. For storage, PENTHROX combination pack should be kept in a locked cabinet, and should not be left on an open shelf.
6.5 Nature and contents of container
PENTHROX is supplied in the following presentations:
3 mL bottle with a tear off tamper-evident seal (packs of 10)
Combination pack with one 3 mL bottle, one PENTHROX Inhaler and one Activated Carbon (AC) chamber (packs of 1 or 10).
Not all pack sizes may be marketed.
6.6 Special precautions for disposal of a used medicinal product
After loading the PENTHROX Inhaler, replace cap onto PENTHROX bottle. After use, place used PENTHROX Inhaler and used bottle in plastic bag provided, seal and dispose of responsibly.
7
MARKETING AUTHORISATION HOLDER
Medical Developments UK Limited c/o Price Bailey LLP Causeway House 1 Dane Street, Bishop’s Stortford Herts CM23 3BT United Kingdom
8
9
27/05/2016