Peritoneal Dialysis Solution Capd/Dpca 2
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Peritoneal Dialysis Solution CAPD/DPCA 2 (CAPD/DPCA)
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
|
1 litre contains: | |
|
CAPD/DPCA 2 | |
|
Sodium chloride |
5.786 g |
|
Sodium (L)- lactate |
3.925 g |
|
Calcium chloride 2 H2O |
0.2573 g |
|
Magnesium chloride 6 H2O |
0.1017 g |
|
Glucose monohydrate |
16.5 g |
|
A anhydrous glucose |
15.0 g |
|
Na + |
134 mmol/l |
|
Ca ++ |
1.75 mmol/l |
|
Mg++ |
0.5 mmol/l |
|
C1- |
103.5 mmol/l |
|
Lactate |
3 5 mmol/l |
|
Theoretical Osmolarity |
358 mOsm/l |
pH ~ 5.5
3 PHARMACEUTICAL FORM
Solution for peritoneal dialysis
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
For use in patients with end-stage chronic renal failure of various genesis.
4.2 Posology and method of administration
CAPD/DPCA is exclusively indicatede for intraperitoneal use.
The mode of therapy, frequency of administration and dwell time required will be specified by the attending physician.
Continuous ambulatory peritoneal dialysis (CAPD)
Unless otherwise prescribed, 2000 ml of solution are infused per treatment. If at the beginning of treatment, pain due to abdominal distension occurs, the dose per treatment can be temporarily reduced to 500 - 1500 ml.
Children should be given doses between 500 and 1500 ml (30 - 40 ml/kg body weight) per treatment, depending on age, height and weight.
In large adults and/or patients who tolerate a larger filling volume, even 2500 or even 3000 ml may be used.
Automated peritoneal dialysis (APD)
If the dose is given by a machine as in intermittent or continuous cycling peritoneal dialysis, the use of larger volume bags is recommended. For this, the sleep safe system or the safe lock system is used.
Method and duration of use:
Dwell times of 4 - 8 hours, a dose of 2000 ml of solution is used four times in every 24 hour period (total dose 8000 ml), or smaller or larger doses as appropriate (dose given merely as a guide, dosage must be adjusted for the individual patient).
Warm the ready-to-use solution to body temperature and then infuse into the peritoneal cavity through a peritoneal dialysis catheter, administration time per dose 5-20 minutes. The dose remains in the peritoneal cavity for 4-8 hours (equilibration time) and is then drained and replaced.
Depending on the fluid status and electrolyte concentration, CAPD/DPCA 2 is used in combination with another peritoneal dialysis solution of higher glucose concentration (i.e. of higher osmolarity) or other potassium or sodium concentrations.
Treatment is carried out on a daily basis using the specified dosage. It is continued for as long as renal replacement therapy is required.
Please read the instructions for use in section 6.6 of this SPC.
4.3 Contraindications
For this specific solution:
Hypokalaemia, hypercalcaemia.
For peritoneal dialysis in general:
Peritoneal dialysis must not be commenced in the presence of one or more of the following diseases:
- Diseases which have an effect on the integrity of the abdominal wall or of the peritoneal cavity, such as: recent abdominal injury, abdominal burns, other extensive inflammatory conditions of the abdominal skin (dermatitis) in the region of catheter exit site, peritonitis; abdominal perforation; a history of abdominal operations with fibrous adhesions; inflammatory bowel diseases (Crohn’s disease, ulcerative colitis, diverticulitis), intra-abdominal tumours, recent abdominal surgery, ileus, abdominal hernias; internal or external abdominal fistulae;
- Pulmonary diseases, especially pneumonia;
- Sepsis;
- Lactacidosis
- Cachexia and extreme weight loss, particularly when adequate nutrition is impossible;
- In rare cases of uraemia which can no longer be managed by peritoneal dialysis;
- Extreme hyperlipidaemia
- In patients who are physically or mentally incapable of performing
peritoneal dialysis as instructed by the physician.
4.4 Special warnings and precautions for use
Electrolyte imbalance, e.g. caused by vomiting or diarrhoea, may necessitate the temporary use of a potassium-containing peritoneal dialysis solution.
In hypercalcaemia, e.g. resulting from high doses of calcium-containing phosohate binders and/or vitamin D, a temporary or permanent change to a solution with a lower calcium content may be necessary.
The treatment volume should be reduced in children, according to age, height and body weight (see also Posology). In the elderly, the increased incidence of hernias should be considered before beginning treatment.
Exact records of fluid balance and body weight must be kept to avoid dehydration or hyperhydration with potentially life-threatening consequences. Regular monitoring of physical findings, electrolytes, creatinine and urea concentrations, serum protein, the level of blood sugar and if necessary, other laboratory parameters (e.g. blood gases, acid-base balance) is also essential.
It is important that treatment regimes are adhered to, if bag exchanges have been carried out too frequently, states of dehydration and/or disorders of blood electrolyte content (electrolyte imbalance) can occur.
In diabetics, the daily insulin dose must be adjusted to take account of the increased glucose uptake. Regular checks on blood glucose are therefore required.
Aseptic conditions must be maintained during dialysate exchange in order to reduce the risk of infection.
Plastic containers may occasionally become damaged during transport from the manufacturer to the dialysis centre or during storage in the hospital. This can result in fungiform or bacterial contamination, with growth of microorganisms in the dialysis solution. All containers should therefore be carefully inspected for damage prior to connection and use of the solution for peritoneal dialysis. Any damage, however minor, to the closure, container welds or corners, must be noted, because of possible contamination.
Bags with cloudy contents must never be used. Only use the peritoneal dialysis solution if the container and closure exhibit no signs of damage. The material should be set aside for examination, including a bacteriological investigation, if necessary.
Only use CAPD/DPCA 2 if the solution is clear and the container is undamaged. Any unused portion of the solution is to be discarded. The peritoneal dialysis solution CAPD/DPCA 2 is not to be used for intravenous infusion.
4.5 Interaction with other medicinal products and other forms of interaction
As a general principle, it should be borne in mind that medication given concurrently might pass into the peritoneal dialysis solution and be lost from the body; hence its dosage may require adjustment.
The possibility of hypercalcaemia should be considered on concomitant administration of calcium compounds or Vitamin D.
Simultaneous administration of diuretics may be worthwhile to support residual excretion by the kidneys, but can also cause disturbances of fluid and electrolyte balance. Potassium levels must be monitored particularly closely during concurrent digitalis therapy, as the sensitivity to these drugs is increased in patients with hypokalaemia.
4.6 Pregnancy and lactation
Peritoneal dialysis treatment should only be carried out in the later stages of pregnancy after careful weighing of the benefits and risks.
4.7 Effects on ability to drive and use machines
When CAPD/DPCA 2 is used as directed, it does not impair driving ability or the operation of machines.
4.8 Undesirable effects
CAPD/DPCA 2 is a solution of electrolytes whose composition is essentially similar to that found in human blood.
Possible side effects may result either from the technique of peritoneal dialysis itself or may be induced by the dialysis solution.
Undesirable effects of the procedure:
Very common complications of any peritoneal dialysis therapy, including CAPD/DPCA 2, are peritonitis and infections of the catheter exit site and tunnel. Untreated peritonitis can lead to sepsis. Symptoms of an incipient peritonitis are cloudiness of the dialysate, abdominal pain, and fever.
Pathogens and white blood cell count in the dialysate must be determined; lack of any elevation in leucocytes does not necessarily exclude peritonitis if other symptoms are present. It is essential to institute treatment (intraperitoneal and/or systemic) without delay, being in accordance with the latest state of scientific knowledge and using agents effective against the presumed pathogen, even before culture results are available. Once these are known, therapy should be adjusted appropriately.
A relative loss of proteins (5-15 g/day) and amino acids (1.2-3.4 g/day) is unavoidable, likewise water-soluble vitamins may also be lost. A deficiency of these substances must be countered by adequate dietary intake.
Hypoproteinaemia may occur if protein intake does not compensate for protein loss.
The transport characteristics of the peritoneal membrane may change during long-term peritoneal dialysis primarily indicated by a loss of ultrafiltration. In severe cases peritoneal dialysis must be stopped and hemodialysis commenced.
In addition, distension and a feeling of fullness (abdominal complaints), in-and outflow disturbances of the dialysis solution, hernia, pain in the shoulder, dyspnoea due to elevation of the diaphragm, diarrhoea, and constipation may occur.
Undesirable effects of the solution:
The dialysis solution may cause fluid and electrolyte imbalance, e.g. hypokalaemia.
Hypercalcaemia can occur if calcium intake is increased, e.g. through concomitant use of calcium -containing phosphate binders. These electrolyte disorders can be corrected by changing to other peritoneal dialysis solutions (hypercalcaemia) or a changed diet (hypokalaemia)
In terms of fluid balance, dehydration or hyperhydration may develop. Severe dehydration, (especially on treatment with solutions of higher glucose concentration) may take the form of low blood pressure, increased heart rate, dizziness and muscle cramps; the opposite, hyperhydration, may cause increased body weight, high blood pressure, swollen legs and shortness of breath.
Disorders of lipid metabolism (dyslipoproteinaemia and hyperlipidaemia) may occur or be exacerbated.
Because of the continuous uptake of glucose from the dialysis solution obesity might rarely occur if the diet of the patient is not adapted to the increased caloric load.
4.9 Overdose
To date, no emergency situations specific to this solution have been reported.
Excessive inflow of dialysis solution can be easily drained into an empty bag. If an exchange has been forgotten, then as a rule the dwell times of the next bags should be reduced in such a way that the total amount of dialysis solution required per day (e.g. 4 x 2000 ml) is still achieved. Incorrect balancing can lead to hyper- or dehydration and electrolyte disturbances.
The most likely consequence of an overdosage with CAPD/DPCA 2 is dehydration. Underdosage or discontinuation of treatment can lead to life-threatening hyperhydration with peripheral oedema and cardiac decompensation and/or other symptoms of uraemia, which may endanger life.
The generally accepted rules of emergency and intensive medicine are applied. The patient may require urgent haemodialysis.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group Group: Solution for peritoneal dialysis
ATC: B05D B
5.2 Pharmacokinetic properties
CAPD uses the peritoneum as a diffuse membrane through which substrate and water can be eliminated from the blood.
Sodium in the solutions is slightly below normal physiological levels leading to a net loss per day. Renal patients are trained to use a low sodium diet which contains about 150 mmol sodium/day.
Potassium levels are either 2 mmol/l or potassium free. This allows the physician to monitor serum potassium levels and adjust the potassium level to prevent hypercalaemia often seen in end stage renal disease. The magnesium content is kept constant at 0.5 mmol throughout the solutions to prevent deficiency.
Dextrose is used to adjust the osmolarity of the solutions from approximately 350 mosmol/kg in CAPD 2 to circa. 525 in CAPD 3K.
Sodium Lactate is used to buffer the solutions at pH of 5.5. Aluminium which has been shown to cause CNS effects is restricted to less than 10pg/litre in line with EEC guidelines.
5.3 Preclinical safety data
Peritoneal dialysis solutions such as CAPD 2 have been in clinical use for many years without any sign of major risk of acute or chronic toxicity and without any indication of mutagenicity, genotoxicity, teratogenicity and cancerogenicity from clinical experience. As all ingredients of the dialysis solution are physiological substances formal toxicological studies seem not to be mandatory.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Hydrochloric acid Sodium hydroxide Water for injections
6.2 Incompatibilities
Because of the risk of incompatibility and of contamination drugs must only be added on prescription by a physician. After thorough mixing and checking for the absence of any turbidity the peritoneal dialysis solution must be used immediately (no storage).
6.3 Shelf life
2 years
6.4 Special precautions for storage
Do not store above 25°C. Do not refrigerate or freeze.
CAPD / DPCA 2 should be stored out of the reach of children.
6.5 Nature and contents of container
Standard
The Standard System is provided as single bag system consisting of a PVC solution bag, a Safe lock connector of polycarbonate and an injection port made of polycarbonate/synthetic rubber.
500ml bags are provided, 10 of them packed in a carton.
1000ml and 750ml bags are provided, 8 of them packed in a carton.
1500 ml bags are provided, 6 of them packed in a carton.
2000ml and 2500ml bags are provided, 4 of them packed in a carton.
A.N.D.Y P.L.U.S.:
The A.N.D.Y. P.L.U.S. system is provided as double bag system consisting of a PVC solution bag with a connecting system of PVC/Polycarbonate, a male connector of polycarbonate/silicone and a drainage bag.
500ml bags are provided, 10 of them packed in a carton.
1000ml and 750ml bags are provided, 8 of them packed in a carton.
1500 ml bags are provided, 6 of them packed in a carton.
2000ml and 2500ml bags are provided, 4 of them packed in a carton.
Stay. Safe:
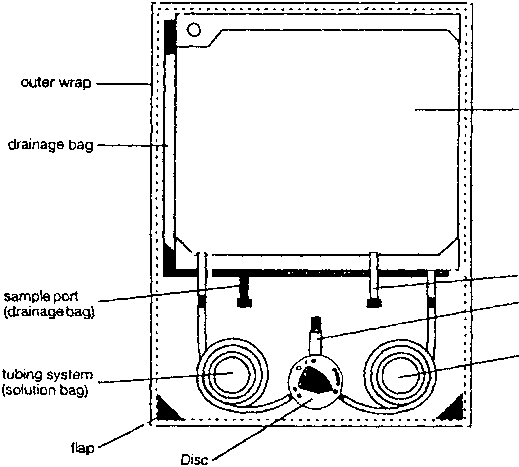
The Stay.Safe system is provided as a double bag system consisting of a non-PVC solution bag made of a multi layer polyolefine foil, a tubing system also made of polyolefines, system connector (DISC) with a rotable switch (polyproylene), and a drainage bag, also made of polyolefine multi layer film
1000ml and 750ml bags are provided, 8 of them packed in a carton.
1500 ml bags are provided, 6 of them packed in a carton.
2000ml and 2500ml bags are provided, 4 of them packed in a carton.
5000ml are provided, 2 of them packed in a carton.
Slee. Safe
The Sleep Safe system is provided as non-PVC solution bag made of a multilayer polyolefine foil, with connectors, injection port and a tubing system also made of polyolefines which has an integrated ambulant peritoneal dialysis bag (APD) connector.
5000ml bags are provided, 2 of them packed in a carton.
Safe Lock
The Safe lock system is provided as non-PVC solution bag made of a multilayer polyolefine foil, with connectors, injection port and a tubing system also made of polyolefines which has an integrated ambulant peritoneal dialysis bag (APD) connector. The solution bag has a fill volume of 5000, 6000 ml.
6.6 Special precautions for disposal
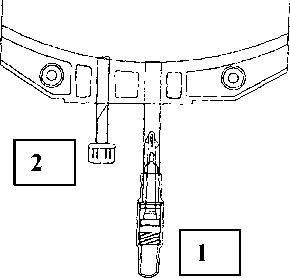
Instructions for use of the Safe lock connector:
1. Remove protective cap of the connector (1) from the connecting line.
2. Connect lines to the bag.
3. Break the inner lock by bending the line and the pin by more than 90° to both sides.
4. The bag is now ready for use.
A separate injection can be effected through the second injection port (2).
Instructions for use of the Andy P.L.U.S. system:
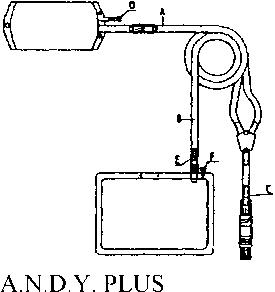
INSTRUCTION FOR USE
1. Grasp the overwrap at the tearing notch with both hands and tear off the overwrap.
2. Remove the patient connector from its packed position within the rolled drainage bag.
3. Remove the ring from the rolled drainage bag and unroll it.
4. Take hold of the tubing at points A (next to the bag) and B (next to the drainage bag) and pull apart to un- wind the spiralled transfer set.
5. Place an external clamp on the inflow line (between filled bag and Y-piece).
6. Remove the protective cap from the patient connector.
7. Connect the line to the catheter connector.
8. Break the pin of the breaking cones (C) and (E) by bending the pin by more than 90° to both sides.
9. Release the clamp on the catheter extension to allow drainage.
10. Following drainage, close the clamp on the catheter extension.
11. Release the clamp on the inflow line to allow the fresh solution to flush any air bubbles from the inflow line to the drainage bag (2 - 3 seconds approx.).
12. Apply an external clamp to the drain line.
13. Release the clamp on the catheter extension to allow inflow.
14. Following inflow, close the catheter clamp.
15. Place the A.N.D.Y. clamp on the pa- tient line between Y-piece and con-nector and close firmly. Break the used system at the Y-piece and dispose of. Separate medications can be in-jected at the injection port (D), and samples can be taken at the sample port (F), using an aseptic technique.
Instruction for use of the stay safe system:
1. Check the solution bag (label, the expiry date and ensure that the solution is clear) - open the outer wrap and package of the disinfection cap.
2. Cleaning hands with an antimicrobial washing solution.
3. Place the DISC into the organizer (suspend solution bag from the upper hole of the infusion pole - unroll the line “solution bag-DISC” - place the DISC into the organizer - afterwards place drainage bag into lower holder of the infusion pole).
4. Place catheter adapter into the organizer.
5. Disinfect your hands and remove protection cap of the DISC.
6. Connect catheter adapter to the DISC.
7. Opening catheter clamp - position “•” - outflow procedure starts.
8. Flush position “•••’’flush of fresh dialysate to the drainage bag (approx. 5 seconds).
9. Inflow - position “o«” - connection between solution bag and catheter.
10. Security step - position “••••” - automated closing of the catheter adapter with the PIN.
11 .Disconnection (remove catheter adapter from DISC part) - screw catheter adapter to the new disinfection cap.
12. Close the DISC with the open end of the protection cap (which is placed in the right hole of the organizer).
13. Check the drained dialysate and disposal.
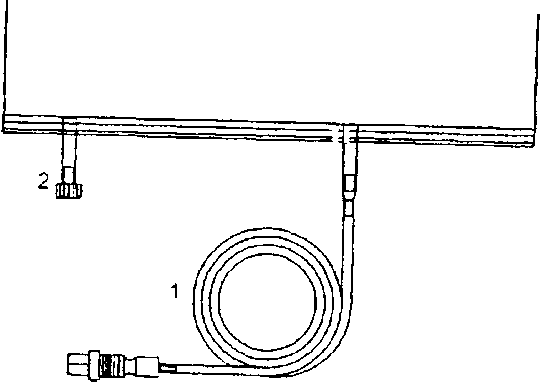
Instruction for use of the sleep •safe system:
1. Unroll tubing (1) of bag.
2. Insert connector in free sleep safe™ tray port.
3. The bag is now ready for use with the steep safe™ set.
A separate injection can be effected through the second injection port (2).
Instructions for use of the Safe lock system:
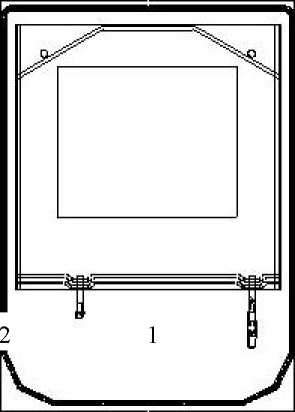
1. Remove protective cap of the connector (1) from the connecting line.
2. Connect lines to the bag.
3. Break the inner lock by bending the line and the pin by more than 90 ° to both sides.
4. The bag is now ready for use.
A separate injection can be effected through the second injection port (2).
7 MARKETING AUTHORISATION HOLDER
Fresenius Medical Care (UK) Ltd
Nunn Brook Road
Huthwaite
Sutton-in-Ashfield
Nottinghamshire
NGI7 2HU
8 MARKETING AUTHORISATION NUMBER(S)
PL 15634/0001
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
18 December 1998
10
DATE OF REVISION OF THE TEXT
08/09/2011