Piroxicam 0.5% W/W Gel
Package leaflet: Information for
the patient
1. What Feldene Gel is and what it is used for
Feldene® 0.5% w/w Gel
(piroxicam)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
See section 4.
Your medicine is known by the above name, but will be referred to as Feldene Gel throughout this leaflet. This medicine is also available as Feldene Gel 112 g tube.
What is in this leaflet
1 .What Feldene Gel is and what it is used for
2. What you need to know before you use Feldene Gel
3. Flow to use Feldene Gel
4. Possible side effects
5. Flow to store Feldene Gel
6. Contents of the pack and other information
Feldene Gel is one of a group of medicines called non-steroidal antiinflammatory drugs (NSAIDs). This means it will help to relieve pain and reduce swelling affecting joints and muscles when rubbed into the skin over the affected area.
Feldene Gel is for the treatment of rheumatism, sprains, strains and mild osteoarthritis of joints (knees, wrists, ankles etc).
2. What you need to know before you use Feldene Gel
Do not use Feldene Gel
• If you are allergic to piroxicam or any of the other ingredients of this medicine (listed in section 6), any other form of piroxicam, aspirin or any other medicines used to treat painful joints and muscles. This may have been itching, reddening of the skin or difficulty in breathing.
Warnings and precautions
Talk to your doctor or pharmacist before taking Feldene Gel. Medicines are not always suitable for everyone. Your doctor needs to know before you use Feldene Gel if you suffer from or have suffered in the past from any of the following conditions:
• liver disease
• kidney disease
Potentially life-threatening skin rashes (Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported with the use of piroxicam, appearing initially as reddish target like spots or circular patches often with central blisters on the trunk.
Additional signs to look for include ulcers in the mouth, throat, nose, genitals and conjunctivitis (red and swollen eyes).
These potentially life-threatening skin rashes are often accompanied by flulike symptoms.
The rash may progress to widespread blistering or peeling of the skin.
The highest risk for occurrence of serious skin reactions is within the first weeks of treatment.
If you have developed Stevens-Johnson syndrome or toxic epidermal necrolysis with the use of piroxicam, you must not be re-started on piroxicam at any time.
If you develop a rash or these skin symptoms, seek immediate advice from a doctor and tell them that you are taking this medicine.
NSAIDs, including Feldene Gel, may cause kidney damage or kidney failure.
Children and adolescents
Feldene Gel is not recommended for use in children under 12 years of age.
Other medicines and Feldene Gel
Feldene Gel is not known to interact with any other medicines. Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy, breast-feeding and fertility
If you are trying to become pregnant or being investigated for infertility, withdrawal of Feldene Gel should be considered. Feldene Gel may increase the risk of miscarriage in early pregnancy.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Feldene Gel is not expected to affect your ability to drive or use machines.
Feldene Gel contains propylene glycol
Feldene Gel contains propylene glycol which may cause skin irritation.
3. How to use Feldene Gel
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Feldene Gel is for external use on the skin only.
Pierce the tube by reversing the cap and screwing down to break the seal on the tube.
Apply 3cm (1 %” approximately) of the Gel on the affected area. Use the scale below as a guide. Rub the Gel into the skin until the Gel disappears. Do this three or four times a day.
If there is no improvement in your symptoms, tell your doctor. If the gel is not rubbed in completely, mild but temporary staining of the skin and staining of clothing may occur.
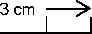
Do not use Feldene Gel for more than four weeks without telling your pharmacist or doctor.
Do not rub your medication into damaged skin, for example wounds, scratches, infections or dermatitis. Do not apply near eyes, nose, mouth, genital or anal (bottom) areas. If the Gel does come into contact with these areas, rinse away with water.
Do not cover the area where Feldene Gel has been rubbed in with dressings or bandages.
After using Feldene Gel
1. Always replace the cap
2. Wash your hands
If you use too much or accidentally swallow Feldene Gel
If you accidentally use too much Feldene Gel this is unlikely to cause any side effects.
If a large amount of your medication is swallowed accidentally, tell your doctor immediately, or contact your nearest hospital casualty department.
If you forget to use Feldene Gel
If you forget to use Feldene Gel, use it as soon as you remember unless it is time for your next application. Do not use double the amount to make up for a missed application.
If you stop using Feldene Gel
Your pain may return if you stop using Feldene Gel.
If you have any further questions on how to take this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately and stop using Feldene Gel if you
experience any of the following symptoms after applying this medicine. Although they are very rare, these symptoms can be serious:
• sudden wheeziness, difficulty in breathing, fever, swelling of eyelids, face or lips, rash or itching affecting the whole body
• potentially life-threatening skin rashes (Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported very rarely (see section 2)
Other side effects that Feldene Gel may cause are:
• Redness, rash and/or itching of the skin where Feldene Gel was rubbed in (for example eczema, contact dermatitis)
• Reaction of the skin to sunlight
Uncommon side effects are:
• Nausea (feeling sick)
• Indigestion
• Stomach discomfort
These effects should disappear if you stop using Feldene Gel. If the discomfort continues tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Feldene Gel
• Do not store above 30°C.
• Keep out of the sight and reach of children.
• Do not use Feldene Gel after the expiry date which is stated on the tube and carton after ‘EXP.’ The expiry date refers to the last day of that month.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• If your medicine have become discoloured or show any other signs of deterioration, consult your doctor or pharmacist who will tell you what to do.
Manufactured by: Farmasierra Manufacturing, S.L., Carretera de Irun, Km 26.200, E-28700 San Sebastian de los Reyes, Spain.
Procured from within the EU & repackaged by PL holder:
Kosei Pharma UK Ltd.,
956 Buckingham Avenue, Slough,
SL1 4NL.
Feldene® Gel is a registered trademark of Pfizer Products Inc.
|POM|
Feldene® 0.5% w/w Gel PL 39352/0279 Leaflet date: 24.10.2014
6. Contents of the pack and other information
What Feldene Gel contains
• Each gram corresponding to 3 cm contains 5 mg of piroxicam.
• Also contains: carbopol, propylene glycol, ethyl alcohol, benzyl alcohol, di-isopropanolamine, hydroxylethylcellulose, purified water.
What Feldene Gel looks like and
contents of the pack
Feldene is a clear pale yellow gel.
Each tube of gel contains 60g of
Feldene Gel.
Piroxicam 0.5% w/w Gel
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
See section 4.
Your medicine is known by the above name, but will be referred to as Piroxicam throughout this leaflet. This medicine is also available as Piroxicam 112 g tube.
What is in this leaflet:
1. What Piroxicam is and what it is used for
2. What you need to know before you use Piroxicam
3. How to use Piroxicam
4. Possible side effects
5. How to store Piroxicam
6. Further information
1. What Piroxicam is and what it is used for
Piroxicam is one of a group of medicines called non-steroidal antiinflammatory drugs (NSAIDs). This means it will help to relieve pain and reduce swelling affecting joints and muscles when rubbed into the skin over the affected area.
Piroxicam is for the treatment of rheumatism, sprains, strains and mild osteoarthritis of joints (knees, wrists, ankles etc).
2. What you need to know before you use Piroxicam
Do not use Piroxicam
• If you are allergic to piroxicam or any of the other ingredients of this medicine (listed in section 6), any other form of piroxicam, aspirin or any other medicines used to treat painful joints and muscles. This may have been itching, reddening of the skin or difficulty in breathing.
Warnings and precautions
Talk to your doctor or pharmacist before taking Piroxicam. Medicines are not always suitable for everyone. Your doctor needs to know before you use Piroxicam if you suffer from or have suffered in the past from any of the following conditions:
• liver disease
• kidney disease
Potentially life-threatening skin rashes (Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported with the use of piroxicam, appearing initially as reddish target like spots or circular patches often with central blisters on the trunk.
Additional signs to look for include ulcers in the mouth, throat, nose, genitals and conjunctivitis (red and swollen eyes).
These potentially life-threatening skin rashes are often accompanied by flulike symptoms.
The rash may progress to widespread blistering or peeling of the skin.
The highest risk for occurrence of serious skin reactions is within the first weeks of treatment.
If you have developed Stevens-Johnson syndrome or toxic epidermal necrolysis with the use of piroxicam, you must not be re-started on piroxicam at any time.
If you develop a rash or these skin symptoms, seek immediate advice from a doctor and tell them that you are taking this medicine.
NSAIDs, including Piroxicam, may cause kidney damage or kidney failure.
Children and adolescents
Piroxicam is not recommended for use in children under 12 years of age.
Other medicines and Piroxicam
Piroxicam is not known to interact with any other medicines. Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy, breast-feeding and fertility
If you are trying to become pregnant or being investigated for infertility, withdrawal of Piroxicam should be considered. Piroxicam may increase the risk of miscarriage in early pregnancy.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Piroxicam is not expected to affect your ability to drive or use machines.
Piroxicam contains propylene glycol
Piroxicam contains propylene glycol which may cause skin irritation.
3. How to use Piroxicam
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Piroxicam is for external use on the skin only.
Pierce the tube by reversing the cap and screwing down to break the seal on the tube.
Apply 3cm (1 %” approximately) of the Gel on the affected area. Use the scale below as a guide. Rub the Gel into the skin until the Gel disappears. Do this three or four times a day.
If there is no improvement in your symptoms, tell your doctor. If the gel is not rubbed in completely, mild but temporary staining of the skin and staining of clothing may occur.
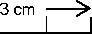
Do not use Piroxicam for more than four weeks without telling your pharmacist or doctor.
Do not rub your medication into damaged skin, for example wounds, scratches, infections or dermatitis. Do not apply near eyes, nose, mouth, genital or anal (bottom) areas. If the Gel does come into contact with these areas, rinse away with water.
Do not cover the area where Piroxicam has been rubbed in with dressings or bandages.
After using Piroxicam
1. Always replace the cap
2. Wash your hands
If you use too much or accidentally swallow Piroxicam
If you accidentally use too much Piroxicam this is unlikely to cause any side effects.
If a large amount of your medication is swallowed accidentally, tell your doctor immediately, or contact your nearest hospital casualty department.
If you forget to use Piroxicam
If you forget to use Piroxicam, use it as soon as you remember unless it is time for your next application.
Do not use double the amount to make up for a missed application.
If you stop using Piroxicam
Your pain may return if you stop using Piroxicam.
If you have any further questions on how to take this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately and stop using Piroxicam if you
experience any of the following symptoms after applying this medicine. Although they are very rare, these symptoms can be serious:
• sudden wheeziness, difficulty in breathing, fever, swelling of eyelids, face or lips, rash or itching affecting the whole body
• potentially life-threatening skin rashes (Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported very rarely (see section 2)
Other side effects that Piroxicam may cause are:
• Redness, rash and/or itching of the skin where Piroxicam was rubbed in (for example eczema, contact dermatitis)
• Reaction of the skin to sunlight
Uncommon side effects are:
• Nausea (feeling sick)
• Indigestion
• Stomach discomfort
These effects should disappear if you stop using Piroxicam. If the discomfort continues tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Piroxicam
• Do not store above 30°C.
• Keep out of the sight and reach of children.
• Do not use Piroxicam after the expiry date which is stated on the tube and carton after ‘EXP.’ The expiry date refers to the last day of that month.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• If your medicine have become discoloured or show any other signs of deterioration, consult your doctor or pharmacist who will tell you what to do.
Manufactured by: Farmasierra Manufacturing, S.L., Carretera de Irun, Km 26.200, E-28700 San Sebastian de los Reyes, Spain.
Procured from within the EU & repackaged by PL holder:
Kosei Pharma UK Ltd.,
956 Buckingham Avenue, Slough,
SL1 4NL.
|POM
Piroxicam 0.5% w/w Gel PL 39352/0279 Leaflet date: 24.10.2014
6. Contents of the pack and other information
What Piroxicam contains
• Each gram corresponding to 3 cm contains 5 mg of piroxicam.
• Also contains: carbopol, propylene glycol, ethyl alcohol, benzyl alcohol, di-isopropanolamine, hydroxylethylcellulose, purified water.
What Piroxicam looks like and
contents of the pack
Feldene is a clear pale yellow gel.
Each tube of gel contains 60g of
Piroxicam.