Pivmecillinam Hydrochloride 200 Mg Film-Coated Tablets
Pharmacode position may change as per Supplier's m/c requirement &additional small pharma code may appear on the front / back panel


Package leaflet: Information for the user
Pivmecillinam hydrochloride 200 mg film-coated tablets
pivmecillinam hydrochloride
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Pivmecillinam hydrochloride is and what it is used for
2. What you need to know before you take Pivmecillinam hydrochloride
3. How to take Pivmecillinam hydrochloride
4. Possible side effects
5. How to store Pivmecillinam hydrochloride
6. Contents of the pack and other information
1. What Pivmecillinam hydrochloride is and what it is used for
Pivmecillinam belongs to a group of medicines called penicillins. It is a type of antibiotic.
Pivmecillinam works by killing germs (bacteria) that cause infections.
Pivmecillinam hydrochloride is used to treat infections of the bladder (cystitis) in adults.
2. What you need to know before you take Pivmecillinam hydrochloride
Your doctor may have prescribed a different use or dosage than stated in this information. Always follow your doctors instructions.
Do not take Pivmecillinam hydrochloride
• Ifyou are allergic to pivmecillinam or any of the other ingredients of this medicine (listed in section 6).
• Ifyou are allergic to other antibiotics. Such antibiotics include penicillin or cephalosporins.
• Ifyou have narrowing of the food pipe.
• Ifyou suffer from a condition which may reduce the amount of a substance called carnitine in your body. Such conditions include carnitine transporter defect, methylmalonic aciduria and propionic acidaemia
Warnings and precautions
Talk to your doctor or pharmacist before taking Pivmecillinam hydrochloride. The tablets must be taken with at least half a glass of fluid to prevent that the tablets get stuck in your food pipe.
Before you take Pivmecillinam hydrochloride tell your doctor:
• Ifyou have porphyria.
• Ifyou have already used Pivmecillinam hydrochloride recently, as use for a longer period may reduce the amount of carnitine in your body.
During treatment:
• Ifyou suddenly have diarrhoea while taking Pivmecillinam hydrochloride tell your doctor immediately. This may be because your bowel is inflamed (colitis).
Children and adolescents
Do not give this medicine to children and adolescents below 18 years because the potential benefits may not be greater than the risks.
Other medicines and Pivmecillinam hydrochloride
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Tell your doctor or pharmacist if you use any of the following medicines:
• Probenecid (for treating gout). This may increase the effect of Pivmecillinam hydrochloride.
• Methotrexate (for treating rheumatism, cancer). The excretion of methotrexate from your body may be slower when used together with Pivmecillinam hydrochloride.
• Valproate or valproic acid (for treating epilepsy). This increases the risk of too little carnitine in your body when used together with Pivmecillinam hydrochloride.
• Other antibiotics (e.g. tetracyclines, erythromycin, other beta-lactam antibiotics). This can influence the effect. of Pivmecillinam hydrochloride.
Pivmecillinam hydrochloride with food and drink
You can take your medicine with or immediately after a meal. You must take Pivmecillinam hydrochloride with at least half a glass of water. It is important to take your medicine with plenty of liquid and in good time before bedtime. This will stop you getting any problems in your food pipe.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Pivmecillinam hydrochloride can be taken during pregnancy. Breast-feeding
Pivmecillinam hydrochloride can be taken during breast-feeding. Fertility
• Clinical studies concerning fertility have not been done.
Driving and using machines
Pivmecillinam hydrochloride has no or negligible influence on your ability to drive or use machines.
3. How to take Pivmecillinam hydrochloride
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you. Check with your doctor, pharmacist if you are not sure. You should take the medicine at equally spaced times in the day. When you have to take it three times a day, take it at breakfast, at lunch and then at dinner time. Pivmecillinam hydrochloride must be taken with at least half a glass of liquid. Your doctor will tell you how long you need to use Pivmecillinam hydrochloride tablets.
The usual dose is 200 mg two times daily for 7 days, alternatively 200 mg 3 times daily for 5 days.
Paediatric population:
Pivmecillinam hydrochloride should not be used in children and adolescents below 18 years because the efficacy and safety have not yet been established.
Elderly: Dose adjustment is not necessary.
If you have reduced kidney function or reduced liver function dose adjustment is not necessary.
Take this tablet as follows:
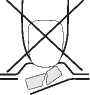
1. Do not push the tablet
Do not push against the tablet pocket.
P150xxxxx

2. Tear off one tablet pocket
Bend and tear off one tablet pocket along the dotted lines.
iO-.O-.O-.O-.O'
'qV o\o\o\o'
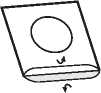
3. Peel off the lid
Lift, hold and peel off the lidding foil, starting from the side indicated by the arrow.
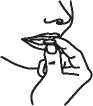
4. Remove the Tablet with dry hands and take as directed above.
If you take more Pivmecillinam hydrochloride than you should
Tell your doctor straight away. You may need to stop taking this medicine.
You may be sick, or feel sick, or get an upset stomach.
If you forget to take Pivmecillinam hydrochloride
If you forget to take your medicine, take it as soon as you remember. Always take it with at least half a glass of water or other liquid. Then take the next dose at the usual time.
If you stop taking Pivmecillinam hydrochloride
It is very important to take all the medicine that your doctor has told you to take. You must finish this medicine even if you feel better. You must do this because otherwise you may feel ill again.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although
not everybody gets them.
The most serious side effects:
Uncommon: may affect up to 1 in 100 people.
• Sudden skin rash, the face and/or the throat swell, breathing difficulty or fainting due to hypersensitivity (anaphylactic reaction). Seek medical help immediately as you may need urgent medical treatment.
• Severe and lasting bloody diarrhoea with stomach pain and fever (pseudomembranous colitis). Seek medical help immediately as you may need urgent medical treatment.
• Bleeding from skin and mucous membranes and bruising of the skin due to changes in the blood (low platelet count) (thrombocytopenia). Contact your doctor.
Other side effects:
Common: may effect upto 1 in 10 people
• Fungal infection of the vagina Feeling.
• Diarrhoea.
• Feeling sick (nausea).
Uncommon: may effect upto 1 in 100 people
• Headache
• Dizziness
• Being Sick (Vomoting)
• Stomach Pain
Mouth or food pipe ulcer, inflammation of the food pipe Liver function disorders Changes in blood or liver values
Muscle weakness or muscle loss. Tiredness or lack of energy.
Too little carnitine in your body may cause these symptoms.
Nettle rash
Itching
Fatigue
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Pivmecillinam hydrochloride
Keep this medicine out of the sight and reach of children.
Store below 30°C.
Store in the original package in order to protect from moisture.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Pivmecillinam hydrochloride contains
- The active substance is pivmecillinam hydrochloride. Each film-coated tablet contains 200 mg of pivmecillinam hydrochloride.
- The other ingredients are:
Tablet core: Cellulose microcrystalline, magnesium stearate. Film coating: Hypromellose, triacetin.
What Pivmecillinam hydrochloride looks like and contents of the pack
Film-coated tablet.
White to off-white, round shaped, biconvex, film coated tablets debossed with 'F' on one side and '48' on the other side.
Pivmecillinam hydrochloride film-coated tablets are available in PVC/OPA/aluminum/PVC/Paper/PET/aluminum foil blister pack.
Pack sizes: 2, 10, 14 and 100 film-coated tablets
Not all pack sizes may be marketed
Marketing Authorisation Holder
Milpharm Limited
Ares Block, Odyssey Business Park West End Road Ruislip HA4 6QD United Kingdom
Manufacturer
APL Swift Services (Malta) Limited HF26, Hal Far Industrial Estate, Hal Far Birzebbugia, BBG 3000 Malta
or
Milpharm Limited
Ares Block, Odyssey Business Park West End Road Ruislip HA4 6QD United Kingdom
This leaflet was last revised in 09/2015.

