Plenaxis 100 Mg Powder And Solvent For Suspension For Injection
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
PLENAXIS® 100 mg powder and solvent for suspension for injection
abarelix
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What PLENAXIS is and what it is used for
2. Before you take PLENAXIS
3. How to take PLENAXIS
4. Possible side effects
5. How to store PLENAXIS
6. Further information
1. WHAT PLENAXIS IS AND WHAT IT IS USED FOR
PLENAXIS is an injectable medicinal product which belongs to the gonadotropin-releasing hormone antagonist class of medicines (GnRH antagonists). In men, this class of medicines leads to a decrease in male hormones, including testosterone. Prostate cancer is treated by reducing the testosterone level.
PLENAXIS is used to initiate (up to 85 days of treatment) medicinally a reduction in the testosterone level in advanced or metastasising, hormone-dependent prostate carcinoma, when androgen suppression (suppression of the male sex hormone) is necessary.
2. BEFORE YOU TAKE PLENAXIS Do not take PLENAXIS
- if you are allergic (hypersensitive) to abarelix or any of the other ingredients of PLENAXIS.
- if you have any problems with your liver and/or kidney function.
Take special care with PLENAXIS
- After every PLENAXIS injection, you should remain at the doctor’s for at least 30 minutes, so that you can be kept under observation and appropriate measures can be taken in the event of an immediate-type allergic reaction.
- Since PLENAXIS may cause changes in an ECG, the doctor should weigh carefully the risks involved in using PLENAXIS against the benefits of treatment, especially if your so-called QTc values are longer than 450 milliseconds.
- Your serum transaminase values (a type of liver function test) will be ascertained by the doctor before treatment begins, as well as periodically during treatment with PLENAXIS. If the values ascertained are markedly higher than normal, your doctor must carry out additional investigations before any further treatment.
- PLENAXIS should not be administered to children.
Pregnancy and breast-feeding
PLENAXIS must not be administered to women.
Using other medicines
No studies of the interaction of PLENAXIS with other medicinal products have been carried out, and no interactions have been observed up to now.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Driving and using machines
Tiredness and dizziness immediately following administration of PLENAXIS are common side effects that may impair your ability to drive and use machines. These effects may be due to the treatment or effects resulting from the underlying disease.
Important information about some of the ingredients of PLENAXIS
PLENAXIS contains sodium, but less than 1 mmol (23 mg) of sodium per dosage unit, which means that it is virtually “sodium-free”.
3. HOW TO TAKE PLENAXIS
PLENAXIS is administered by a doctor or nurse in the form of an injection in the buttock muscle. During the first month, this takes place on Day 1, Day 15, Day 29 (week 4) and subsequently once every four weeks. It is important that you make an appointment with your doctor on the date on which your injection is due. Ensure that your treatment with PLENAXIS is continued, even when you feel well, until your doctor decides that treatment can be ended.
If treatment lasts for longer than three months, your body’s response to treatment with PLENAXIS must be closely checked, because its efficacy decreases between the 3rd and 12th month in some patients and it has also not yet been adequately investigated for a period in excess of 12 months. Particular attention should be paid to this in patients with a body weight > 100 kg. The success of PLENAXIS therapy can be monitored by means of certain clinical signs and the periodic measurement of serum testosterone and PSA levels.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, PLENAXIS can cause side effects, although not everybody gets them.
PLENAXIS can cause allergic reactions leading to a drop in blood pressure and fainting fits. Since these reactions occur shortly after the administration of an injection, your doctor should monitor you for a period of 30 minutes after the injection. Should you feel weak at another time shortly after an injection, seek medical assistance immediately.
Since PLENAXIS affects the testosterone level, other side effects can generally be attributed to a change in hormonal balance. These side-effects may include the following: swelling and sensitivity of the breast, hot flushes, weight gain, depression, impotence, reduced sexual desire and sleep disturbances. Some men may suffer pain or a change in the size of the testicles, as well as changes in passing water. Additional frequent side-effects are: pain at the injection site, tiredness, weakness, dizziness, headache, tingling sensation, rash and itching. In addition, disturbance of the hormonal balance may also be connected with gastrointestinal
symptoms such as stomach ache, nausea, diarrhoea or constipation, and flatulence. In certain circumstances, regular checks of liver function are necessary.
The following frequencies were used as a basis in assessing undesirable effects:
Very common: more than 1 in 10 treated Common: less than 1 in 10 but more than 1 in 100 treated Uncommon: less than 1 in 100 but more than 1 in 1,000 treated Rare: less than 1 in 1,000 but more than 1 in 10,000 treated Very rare: less than 1 in 10,000 treated, or unknown
|
System Organ Class |
Very Common (>1/10) |
Common (>1/100 to <1/10) |
Uncommon (>1/1,000 to <1/100) |
|
Blood and lymphatic system disorders |
Abnormal blood clotting, anaemia. | ||
|
Cardiac disorders |
Heart enlarged, abnormal heart rhythm (fast or slow), palpitations, disease of the blood vessels of the heart. | ||
|
Vascular disorders |
Hot flushes. |
Nose bleed, bruising, high blood pressure, flushing, low blood pressure. | |
|
Ear and labyrinthine disorders |
Ear pain. | ||
|
Eye disorders |
Cataracts, eye abnormalities, sight impairment, dry eyes. | ||
|
Gastrointestinal disorders |
Stomach pain, vomiting, constipation, flatulence, diarrhoea, stomach distension. |
Inflammation of the lining of the abdominal cavity, sickness, dry mouth, indigestion, frequent bowel movements, faecal incontinence, rectal bleeding, haemorrhoids, inflammation of the gums, toothache, inflammation of the lower bowel. | |
|
General disorders and administration site conditions |
Weakness. |
Injection site pain, swelling, chest pain. |
Injection site bruising and inflammation, pain, tiredness, raised temperature, swelling. |
|
Immune system disorders |
Allergic reactions, itching. |
Itchy rash. | |
|
Infections and Infestations |
Shingles, bladder infection. | ||
|
Injury, poisoning and investigational complications |
Other injuries. | ||
|
Investigations |
Liver enzyme increased, weight gain. |
Changes in blood chemistry, weight loss, heart murmur, abnormal |
|
breathing sounds. | |||
|
Metabolic and nutrition disorders |
Increased appetite, abnormal blood fats, worsening of diabetes, gout. | ||
|
Musculoskeletal and connective tissue disorders |
Muscle weakness, muscle pain, leg pain. |
Back pain, joint pain, inflamed joints, muscle wasting. | |
|
Nervous system disorders |
Dizziness, headaches, pins and needles. |
Disturbed: balance, and coordination, speech, walking touch sensation, leg cramps, increased migraine, face pain, fainting. | |
|
Psychiatric disorders |
Anorexia, depression, abnormal sleep patterns, drowsiness. |
Agitation, anxiety, memory loss, concentration loss, confusion, worsening of depression, abnormal dreams, abnormal mood, nervousness, neurosis. | |
|
Renal and urinary disorders |
Overactive bladder, urge to pass urine during the night, painful urination. |
Abnormalities of passing urine including: incontinence, reduced flow, pain, inability to pass urine and blood in the urine. | |
|
Reproductive system and breast disorders |
Development of breasts. |
Nipple pain, erectile dysfunction, reduced sexual interest, testicular pain, testicular disorders. |
Painful testicles, disorders of the penis and prostate. |
|
Respiratory system, thoracic and mediastinal disorders |
Breathlessness. |
Bronchitis, coughing, catarrh, fluid on the lung, increased mucus of nose and throat, sinus and upper airway inflammation. | |
|
Skin and subcutaneous tissue disorders |
Hair loss, rash. |
Acne, rash, dermatitis, reddening of the skin, dry skin, genital itching, hair disorder, increased sweating, chills, skin nodules, skin reaction. |
Treatment with PLENAXIS over a long period can lead to a reduction in bone mineral density.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE PLENAXIS
Keep out of the reach and sight of children.
You may be given a prescription for obtaining PLENAXIS from a pharmacy. You should not obtain the prescription until shortly before your next visit to the doctor. Keep PLENAXIS in a safe place in its original packaging until your next treatment.
PLENAXIS does not require any special storage conditions.
Do not use PLENAXIS after the expiry date which is stated on the vials and the carton after EXP (abbreviation used for expiry date). The expiry date refers to the last day of that month.
After reconstitution
Chemical and physical in-use stability has been demonstrated for 8 hours at 25°C.
From a microbiological point of view, unless the method of reconstitution precludes the risk of contaminiation, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION What PLENAXIS contains
The active substance in PLENAXIS is abarelix.
The other ingredient in Plenaxis® is the solvent. The solvent is sodium chloride 9 mg/mL (0.9%) solution for injection.
What PLENAXIS looks like and contents of the pack
PLENAXIS is supplied as a kit and contains:
• A glass vial containing a whitish powder.
• An ampoule containing 3 ml of a clear, colourless solvent (sodium chloride 9 mg/mL (0.9%) solution for injection) for suspension for injection.
• In addition, a syringe and 2 needles for reconstitution and administration.
Package sizes:
Kit for 1 injection Kit for 3 injections
Marketing Authorisation Holder
Speciality European Pharma Limited
14 Took’s Court
London
EC4A 1LB
United Kingdom
Tel.: +44(0) 20 7421 7400
Manufacturer
Fisher Clinical Services UK Limited
Langhurstwood Road
Horsham
West Sussex
RH12 4QD
United Kingdom
Tel.: +44 (0) 1403 212 700
Chester Medical Solutions
3-4 Apex Court
Bassendale Road
Bromborough
Wirral
CH62 3RE
United Kingdom
Tel.: +44 (0) 151 343 1181
This medicinal product is authorised in the Member States of the EEA under the following names:
Belgium: Plenaxis 100 mg poeder en oplosmiddel voor suspensie voor injectie / Plenaxis 100 mg de poudre et solvant pour suspension injectable / Plenaxis 100 mg Pulver und Losungsmittel zur Herstellung einer Inj ektionssuspension
Germany: Plenaxis 100 mg Pulver und Losungsmittel zur Herstellung einer Injektionssuspension Luxembourg: Plenaxis 100 mg de poudre et solvant pour suspension injectable / Plenaxis 100 mg Pulver und Losungsmittel zur Herstellung einer Injektionssuspension
Netherlands: Plenaxis 100 mg poeder en oplosmiddel voor suspensie voor injectie United Kingdom: Plenaxis 100 mg powder and solvent for suspension for injection
This leaflet was last approved in 08/2012
Detailed information on this medicine is available on the website of www.specialityeuropeanpharma.com
The following information is intended for medical or healthcare professionals only:
Instructions for use
Important! It is essential to follow these instructions fully to ensure that the full therapeutic dose is administered.
1. Use aseptic technique for all steps.
2. PLENAXIS powder may have aggregated during storage. To ensure that the patient receives the full dose (100 mg), it is critical that PLENAXIS powder be as fine as possible prior to addition of solvent. Hold the vial at an angle of 45° and tap repeatedly on a hard surface or shake vigorously to fully disperse the powder.
Important! Look for clumps of powder by inspecting the vial whilst rolling it back and forth on its side. Do not proceed to the next stage without ensuring that all the lumps have been removed and the contents are in the form of a fine powder.
4.
5.
6.
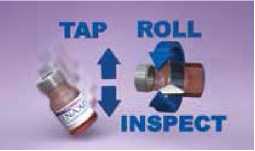
Withdraw 2.2 ml of the solvent (0.9% sodium chloride for injection purposes) using the enclosed syringe and 18G needle. Discard the remaining solvent.
Important! Keeping the vial upright (base down), insert the needle all the way into the vial until it cannot be inserted any further. Position the tip of the needle over the dimple at the base of the vial. Inject the solvent vigorously to ensure maximum dispersion of powder.

Before withdrawing the needle, remove 2.2 ml of air. Now remove the needle from the vial and expel the air.
Important! Do not shake the vial as this causes foaming. Foaming must be avoided to ensure that all the suspension is taken up into the syringe. Tip, roll and swirl the vial for 15 seconds and allow to stand for 2 minutes. During the 2 minutes, tap the vial and swirl it occasionally to reduce any foam.

7.
8.
Repeat step 6 one more time.
Insert the 18G needle back into the vial. Invert the vial and ‘wash’ the walls by withdrawing and gently reinjecting the suspension. Inspect the walls for any particulate matter. Repeat reinjection of
suspension as many times as necessary to ensure all PLENAXIS powder is completely in suspension.

9. Holding the vial at a 45° angle, withdraw the entire contents (at least 2 ml). Do not withdraw the needle until all the PLENAXIS suspension has been drawn into the syringe. There should be no residual lumps or powder on the inner wall of the vial.
10. After withdrawing the needle, pull the syringe plunger back to draw up the residual suspension in the 18G needle into the syringe.
11. Now replace the 18G needle with the supplied 22G needle.
12. Insert the needle at the desired injection site and pull back the plunger to check for back-flow of blood. If blood flows into the syringe, do not inject at this site. Select another injection site.
13. Immediately administer the entire reconstituted suspension intramuscularly.
14. Observe the patient for approximately 30 minutes for any sign of an allergic or hypersensitive reaction.
Unused medicinal products or waste materials should be disposed of in accordance with local requirements.
7