Pliaglis 70 Mg/G + 70 Mg/G Cream
NOTICE RECTO 180x315
PLAN n° E,GD,OE,DRA,035E,R01

70 mg/g + 70 mg/g, cream
Package leaflet: Information for the user
Pliaglis 70 mg/g + 70 mg/g Cream
Lidocaine and Tetracaine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Pliaglis is and what it is used for
2. What you need to know before you use Pliaglis
3. How to use Pliaglis
4. Possible side effects
5. How to store Pliaglis
6. Contents of the pack and other information
1. What Pliaglis is and what it is used for
Pliaglis is a cream containing local anaesthetics, lidocaine and tetracaine which are used to numb an area of skin before a painful procedure such as needle insertion or laser treatments.
2. What you need to know before you use Pliaglis
Do not use Pliaglis
- if you are allergic to lidocaine or tetracaine, any similar local anaesthetics or any of the other ingredients of this medicine (listed in section 6).
- if you know that you are allergic to para-aminobenzoic acid (sometimes called PABA), a substance that is made when your body breaks down tetracaine, methyl parahydroxybenzoate (E218) or propyl parahydroxybenzoate (E216)
- on broken or irritated skin
- on mucous surfaces such as your mouth
Warnings and precautions
Talk to your doctor or pharmacist before using Pliaglis
- if you have problems with your liver, kidney or heart
- if you are very ill or physically weak as you may be more sensitive to Pliaglis
Take care to avoid contact with the eyes. If Pliaglis comes into contact with your eye, immediately rinse your eye with water or salt solution and protect it until feeling returns.
Pliaglis should not be applied for a longer time than recommended. See section 3.
Once Pliaglis has been removed, your skin will feel numb. Take care not to scratch or rub the numbed area or touch very hot or cold surfaces until the numbness has stopped as you could accidently damage the skin.
Children and adolescents
Do not give this medicine to children and adolescents aged up to 18 years because the safety and efficacy have not been established.
Other medicines and Pliaglis
Tell your doctor or pharmacist if you are taking or have recently taken, or might take any other medicines.
The risk of side effects increases if Pliaglis is used at the same time as other medicines, e.g.:
- some medicines for treatment of heart conditions such as quinidine, disopyramide, tocainide, mexiletine and amidarone,
- medicines known to induce methemoglobinemia such as: fonamides, naphthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, and quinine,
- other medicines containing lidocaine and / or tetracaine.
Pliaglis with food and drink
You can use Pliaglis either before or after eating or drinking Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Ask your doctor or pharmacist for advice before using Pliaglis during pregnancy.
Breastfeeding
Breastfeeding can continue while using Pliaglis as long as you do not apply Pliaglis on your breast.
Driving and using machines
Pliaglis has no or negligible influence on your ability to drive and use machines.
Pliaglis contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216).
May cause allergic reactions (possibly delayed).
3. How to use Pliaglis
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is approximately 1.3 g of cream per 10 cm2.
Pliaglis is for single patient use.
Pliaglis should only be applied to dry, intact skin.
Do NOT apply Pliaglis on your face yourself.
Application of Pliaglis on your face should only be done by your physician.
Pliaglis has to be spread evenly and thinly (approximately 1 mm thickness) across the area to be treated (as determined by your doctor) using a flat-surfaced tool such as a metal spatula or tongue depressor. Pliaglis should never be applied with fingers. Do not cover the treated area with an occlusive dressing.
Do not touch the cream with your fingers.
Take care to avoid contact with the eyes. If Pliaglis comes into contact with your eye, immediately rinse your eye with water or salt solution and protect it until feeling returns.
The cream should be left to dry for 30 to 60 minutes depending on the procedure, as determined by your doctor.
After waiting for the required application time the dried cream will have formed a soft peel on your skin. Pliaglis can be removed by grasping a free-edge of the peel and pulling it away from the skin.
The peel should be carefully disposed of immediately after removal (see Section 5 for more information on how to dispose the peel).
Wipe with a compress (such as a tissue or cotton wool) any remaining peel residue from the area.
Hands should be washed immediately after removing and disposal of the peel.
If you use more Pliaglis than you should
The maximum application area should not exceed 400cm2 (no more than two 30g tubes should be used). If too much Pliaglis is used you should tell your doctor or pharmacist immediately.
If you think the amount of Pliaglis is too little, tell your doctor immediately.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects happen where the cream has been placed on the skin. They are generally mild, only last for a short time and usually go away after the end of treatment.
The two medicines that make up Pliaglis (lidocaine and tetracaine) may cause allergic (anaphylactoid) reactions such as skin rash, swelling and breathing difficulties. If you experience any of these side effects, you must remove Pliaglis immediately and contact a doctor.
Most of these side effects occurred on the site of application.
Very common: may affect more than 1 in 10 people
• Redness of the skin
• Skin discolouration
Galderma Laboratories
Product code: P25220-1 Product description: PLIAGLIS CRE Market: GBR / IRL
Article: Leaflet Font size: 8 pt
Flat size: 180x315 Pharmacode: 706
Fold size: 180x26,25 Specification n° MT.09.MPS.1470
PMS 432U
Artwork approved by:
Date:
Signature:
LAUNCHING DEPARTMENT - Z.I. Galderma 74540 ALBY-SUR-CHERAN - FRANCE
|
Surface Area of Treatment Site (cm2) |
Approximate weight of Pliaglis Dispensed (g) | |
|
10 |
1.3 |
2 fingertip units |
|
50 |
6.5 |
Half content of a 15g tube |
|
100 |
13 |
Full content of a 15g tube |
|
200 |
26 |
Full content of a 30g tube |
|
400 |
52 |
Full content of two 30g tubes |
The maximum application area should not exceed 400cm2.
Common: may affect up to 1 in 10 people
• Swelling of the skin
Uncommon side effects: may affect up to 1 in 100 people
• Itchy skin
• Pain or pain of skin
Rare: may affect up to 1 in 1,000 people
• Pallor of the skin
• Skin burning sensation
• Swelling of the face
• Peeling of the skin
• Skin irritation
• Tingling feeling
• Swelling of the eyelid
Not known: frequency cannot be estimated from the available data
• Urticaria
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Ireland
FREEPOST: HPRA Pharmacovigilance, Earlsfort Terrace, IRL -Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517.
Website: www.hpra.ie; E-mail:medsafety@hpra.ie
5. How to store Pliaglis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the tube and carton after “EXP”. The expiry date refers to the last day of that month
Pliaglis should be stored in a refrigerator (2°C -8°C).
Once removed from the refrigerator, store Pliaglis below 25°C for a maximum of 3 months. Do not refrigerate again. Do not freeze. It is recommended that the date of removal from the refrigerator is noted on the packaging.
Shelf life after first opening: 3 months.
Do not use Pliaglis if you notice the packaging is damaged in any way.
Dispose of waste peel with care, since the waste peel contains concentrated quantities of the ingredients. To help protect the environment, do not flush waste peel down the toilet. Waste peel should be put in a closed container, such as a plastic bag. Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Pliaglis contains
- The active substances are lidocaine and tetracaine; 1 gram of cream contains: 70 mg lidocaine and 70 mg tetracaine.
- The other ingredients are calcium hydrogen phosphate anhydrous, purified water, polyvinyl alcohol, paraffin white soft, sorbitan monopalmitate, methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216).
What Pliaglis looks like and contents of the pack
This medicinal product is presented as a white to off white cream.
It will come in a 15 or 30 g tube with a screw cap packed in a cardboard carton.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Galderma (UK) Ltd Meridien House 69-71 Clarendon Road Watford
Herts WD17 1DS - UK
Product Licence Number: PL 10590/0059 (UK) & PA 590/26/1 (IRE) Manufacturer:
Laboratoires Galderma Z.I. - Montdesir 74 540 Alby-sur-Cheran France
This medicinal product is authorised in the Member States of the EEA under the following names:
AT, BE, FR, DE, EL, FI, IE, IT, LU, NL, PL, PT, ES, UK: PLIAGLIS DK, NO: PLIAPEL
This leaflet was last approved in 05/2014.
The following information is intended for medical or healthcare professionals only.
Pliaglis is for single patient use.
For facial procedures, Pliaglis should be applied by healthcare professionals. For procedures on any other part of the body, Pliaglis should be applied by healthcare professionals or by patients adequately instructed in appropriate application technique. Patients and healthcare professionals are recommended to avoid repeated direct contact with the cream or the skin covered with the cream, in order to prevent contact dermatitis. Pliaglis should never be applied with fingers.
Pliaglis should only be applied using a flat-surfaced tool such as a spatula or a tongue depressor.
Hands should be washed immediately after removing and disposal of the peel.
For dermatological procedures such as pulse-dye laser therapy, laser-assisted hair removal, non-ablative laser facial resurfacing, dermal filler injections and vascular access, apply approximately 1.3 g of Pliaglis per 10 cm2 onto intact skin at a thickness of 1 mm for 30 minutes.
For dermatological procedures such as laser-assisted tattoo removal and laser leg vein ablation, apply approximately 1.3 g of Pliaglis per 10 cm2 onto intact skin at a thickness of 1 mm for 60 minutes.
Determine the size of the area to be treated.
The table below can be used as an indication of the quantity of cream to be applied to obtain a 1mm thickness depending on the area to be treated.
1) Spread Pliaglis evenly and thinly (approximately 1 mm thickness) across the area to be treated using a flat-surfaced tool such as a metal spatula or tongue depressor. Take care to avoid contact with the patient’s eyes and your eyes.
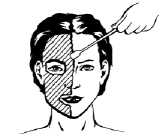
2) Depending on the procedure, the cream should be left to dry either 30 or 60 min.
3) After waiting for the required application time the cream will have formed a pliable peel on the skin. Remove Pliaglis by grasping a free-edge of the peel with your fingers and pulling it away from the skin
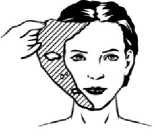
4) Wipe any remaining peel residue from the area and prepare the patient for the procedure. The duration of skin anaesthesia can last from 2 to 13 hours after removal of the peel.
5) Immediately after removal, the peel should disposed of in accordance with local requirements
6) Wash your hands immediately after removing and disposing of the peel.
GALDERMA |
Galderma Laboratories
Product code: P25220-1 Product description: PLIAGLIS CRE Market: GBR / IRL
Article: Leaflet Font size: 8 pt
Flat size: 180x315 Pharmacode: 706
Fold size: 180x26,25 Specification n° MT.09.MPS.1470
PMS 432U
Artwork approved by:
Date:
LAUNCHING DEPARTMENT - Z.I. Galderma 74540 ALBY-SUR-CHERAN - FRANCE
Signature:
GALDERMA DIELINES