Polibar 94 % W/W Powder For Rectal Suspension
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Polibar 94 % w/w powder for rectal suspension
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active Constituent:
Barium sulfate 94.015 % w/w
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder for rectal suspension.
White powder.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
This medicinal product is for diagnostic use only.
Polibar is a diagnostic reagent for radiological examination of the gastrointestinal tract.
Polibar is indicated in adults and children.
4.2 Posology and method of administration
Posology
Polibar is for rectal (enema) administration.
The administered dose of Polibar will depend on the patient in question and the section of the gastrointestinal tract to be viewed.
Adults: Usual Dosage Range 150- 750 g barium sulfate in a suitable suspension.
Double contrast of the large bowel - Give as required between 60 - 115 % w/v. Single contrast of the large bowel - Give as required between 20 - 40 % w/v.
The actual administered dose should be determined, from experience, by the radiologist.
Children: The dosage will be dependent on the size, age, health state and anatomic region to be imaged of the child. Individual requirements should be determined, from experience, by the radiologist.
Elderly: There are no special dosage recommendations. The dosage should be determined, from experience, by the radiologist.
Method of administration
For instructions on reconstitution of the medicinal product before administration, see section 6.6
4.3 Contraindications
Immune System Disorders
Hypersensitivity to barium sulfate or to any of the excipients listed in section 6.1.
Gastrointestinal Disorders Patients with any of the following:
- a known or suspected perforation of the lower gastrointestinal tract
- lower gastrointestinal haemorrhage
- gastrointestinal ischemia
- megacolon or toxic megacolon
- necrotising entercolitis
- severe ileus should not receive Polibar.
Surgical and Medical Procedures
Barium sulfate should not be administered immediately after gastrointestinal surgery, including snare polypectomy or ‘hot’ colonic biopsy because of the potential for post-surgical or post procedural leakage or the potential for gastrointestinal perforation. In case the patient has to undergo gastrointestinal surgery immediately after barium sulfate administration, caution should be exercised.
Injury, Poisoning and Procedural Complications
Barium Sulphate products should not be used during and up to four weeks after radiotherapy to the rectum or prostate. Do not use if there are new injuries or chemical burns of the lower gastrointestinal tract.
4.4 Special warnings and precautions for use
The product should be administered under the supervision of a physician.
Diagnostic procedures which involve the use of radiopaque contrast agents should be carried out under the direction of personnel with the requisite training and with a thorough knowledge of the particular procedure to be performed.
Barium Sulphate should not be administered in its dry form since. The powder must be reconstituted, and some of the commercially prepared suspensions require further dilution, prior to administration.
Hypersensitivity
A history of bronchial asthma, atopy, as evidenced by hay fever and eczema, a family history of allergy, or a previous reaction to a contrast agent warrant special attention.
As stated in section 4.8, serious adverse reactions, including death, have been reported with the administration of barium sulfate formulations and are usually associated with the technique of administration, the underlying pathological condition and/or patient hypersensitivities. Anaphylactic and allergic reactions have been reported during double contrast examinations in which glucagon has been used. Rapid recognition, assessment, and diagnosis are crucial to the effective implementation of treatment. Imaging facilities should be staffed with well-trained personnel for the diagnosis and treatment of hypersensitivity reactions.
Barium sulfate preparations used as radiopaque media contain a number of additives to provide diagnostic properties and patient palatability. Allergic responses following the use of barium sulfate suspensions have been reported. Skin irritation, redness, inflammation and hives have been reported for infants and small children following spillage of barium sulfate suspension on their skin. These responses are thought to be caused by the flavors and/or preservatives used in the product.
Known hypersensitivity or allergy to latex is a contraindication for the use of balloon retention enema tips containing latex.
Because of reported anaphylactoid reactions to latex, the use of non-latex gloves during the procedure should be considered.
Perforation
In patients with a serious stenosis of the lower gastrointestinal tract, and in the presence of conditions and ailments that increase the risk of perforation such as known gastrointestinal fistulae and carcinomas, inflammatory intestinal disease, diverticulitis and diverticulosis and amoebiasis, careful consideration of the risks and benefits of the administration of a barium sulfate suspension is required.
Care must be taken during insertion of the enema tip into the patient and when using a retention balloon, particularly in the newborn, the elderly and in patients with recto-sigmoidal strictures, inflammatory bowel disease, rectal neoplasm or radiation therapy. Enema tip forceful, too deep insertion or balloon inflation may cause tearing or perforation of the rectum.
Insertion of an enema tip should be performed only after digital examination by qualified medical personnel. When balloon retention tips are used, care should be taken to avoid over inflation of the balloon, since overfilling or asymmetrical filling may cause displacement of the tip. Such a displacement can lead to rectal perforation or barium sulfate granulomas.
Inflation of the balloon should be done under fluoroscopic control by qualified medical personnel. Do not unnecessarily move the enema tip once inserted. A specially designed enema tip is required for a barium sulfate suspension examination of a colostomy patient. Intubation of an enteroclysis catheter should be done by qualified medical personnel.
Fluid Overload
Barium sulfate suspensions have been reported to cause fluid overload due to water absorption.
Children and patients with impaired renal function are the most susceptible to water intoxication, as are children with Hirschsprung’s Disease.
It is suggested to not fill the entire colon when evaluating a child with Hirschsprung’s Disease; use only the amount of fluid necessary for the diagnosis.
Preparatory enemas in patients with Hirschsprung’s Disease should be avoided.
Intravasation
Barium sulfate may also intravasate into the venous drainage of the large bowel and enter the circulation as a "barium embolus". This complication occurs rarely, but can lead to potentially fatal complications, including systemic and pulmonary embolism, disseminated intravascular coagulation, septicaemia and prolonged severe hypotension. It is more likely to occur in elderly patients, due to thinning of the rectal wall and vaginal thinning with age, and in those with colorectal disease, when intraluminal pressure overcomes the resistance of the colonic wall affected by colitis, diverticulitis or intestinal obstructionThe diagnosis should be considered in any patient who collapses during or shortly after barium enema, and in those who become suddenly unwell in the hours following the procedure.
This complication may be prevented by ensuring correct placement of the rectal catheter and by reducing the use of balloon catheters.
Misplacement of the rectal catheter in the vagina can lead to intravasation: correct rectal catheter placement should be confirmed prior to enema administration.
Constipation
Polibar should be used with care if the patient is dehydrated, suffers from any condition or is on any other treatment that can cause constipation, or if the patient has history of constipation. In this situation a mild bulk laxative should be administered following completion of the X-ray examination. Increased intake of liquids is recommended after oral or rectal administration of barium sulfate to prevent severe constipation and the risk of impaction.
Other Possible Complications
Care must be taken during an enema procedure as vasovagal reactions, syncopal episodes, cardiac dysrhythmia and other cardiovascular side effects can occur during barium enemas.
All plastic/rubber accessories are disposable, single-use devices that must not be reused or left in the body cavity for an extended period of time.
Apprehensive patients may develop weakness, pallor, tinnitus, diaphoresis and bradycardia following the administration of any diagnostic agent. Such reactions are usually unpredictable and are best treated by having the patient lie flat for an additional 10 - 30 minutes under observation.
Patient preparation for diagnostic gastrointestinal examinations frequently requires cathartics and a liquid diet. The various preparations can result in water loss for the patient. Patients should be rehydrated quickly following a barium sulfate suspension examination of the gastrointestinal tract. In patients with reduced colon motility, saline cathartics may be required after the barium sulfate suspension enema. Saline cathartics are recommended on a routine basis in patients with a history of constipation unless clinically contraindicated.
Baroliths
Baroliths consist of inspissated barium associated with faeces. They are often asymptomatic, but may be associated with abdominal pain, appendicitis, bowel obstruction, or perforation. Patients who are elderly, with impaired gastrointestinal motility, colon obstruction, electrolyte imbalance, dehydration or on a low residue diet may be at risk of developing baroliths. To reduce this risk, adequate hydration should be maintained during and in the days following barium sulfate procedure. The use of laxatives (especially in case of constipation) should be considered.
Children, Elderly and Debilitated Patients
As with any barium sulfate preparation, care should be taken when administering Polibar to children, the elderly or the debilitated. It should be used cautiously in patients with pre-existing heart disease. As bacteraemia may occur during a barium enema, IV antibiotic cover is recommended for patients with prosthetic heart valves.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
Barium sulfate is biologically inert and there are no known interactions with other medicinal products. However, the presence of barium sulfate formulations in the gastrointestinal tract may alter the absorption of therapeutic agents taken concomitantly. In order to minimise any potential change in absorption, the separate administration of barium sulfate from that of other medicines should be considered.
Other examinations of the same area of the gastrointestinal tract with another contrast agent may be complicated by the presence of barium sulfate (residue) in the gastrointestinal tract up to several days following the examination with barium contrast media.
4.6 Fertility, pregnancy and lactation
Pregnancy
Although this product is not contraindicated in pregnancy, we would like to point out that radiographic procedures may damage the foetus, particularly during the first trimester of pregnancy. Any examination should only be carried out after careful consideration of the benefit/risk of the procedure.
Fertility
Following oral or rectal administration, barium sulfate is absorbed systemically in negligible amounts. Though barium sulfate is pharmacologically inert, no studies of its mutagenic or teratogenic potential are available.
Breastfeeding
Since the absorption of barium sulfate is negligible, its use is not contraindicated during breastfeeding.
4.7 Effects on ability to drive and use machines
Polibar has negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Undesirable effects may occur during or after a procedure with barium sulfate.
Skin and subcutaneous disorders together with immune system disorders, reflecting allergic reactions either to barium sulfate or the product excipients, are among the most commonly reported effects; for example urticaria, erythema and rash.
Gastrointestinal disorders are also one of the most frequently reported class of undesirable effects; for example diarrhoea, nausea, abdominal pain/distention, constipation.
Within the table below, clinically significant adverse reactions are listed if they have been reported during post approval use of all barium sulfate formulations. Their frequency is not known, therefore relative reporting rate (for example, less commonly) compared to overall reporting for barium sulfate is used.
|
MedDRA System Organ Class |
Adverse events | |
|
Clinical Trials |
Post-marketing Surveillance | |
|
Rare (>1/10,000 to <1/1,000) | ||
|
Infections and infestations |
Appendicitis, Bacteraemia. Less commonly other infections have been reported including rare cases of Abscess intestinal, Liver abscess, Peritoneal infection and Pneumonia | |
|
Blood and the lymphatic system disorders |
Lymphadenopathy | |
|
Immune system disorders |
Hypersensitivity presenting with a wide range of signs and symptoms including skin and subcutaneous reactions such as urticaria, pruritus, rash, erythema and facial swelling. Potential hypersensitivity associated respiratory signs and symptoms including dyspnoea, pharyngeal oedema and throat tightness have been reported. Anaphylactic reaction and anaphylactic shock have been reported less commonly. | |
|
Metabolism and nutrition disorders |
Infrequent cases of Hyperglycaemia have been reported in diabetic patients | |
|
Psychiatric disorders |
Agitation, Confusional state, Nervousness and related symptoms have been reported during the administration of barium sulfate | |
|
Nervous system disorders |
Loss of consciousness, Syncope, Syncope vasovagal, Dizziness, Burning sensation, Headache, Dysarthria, Hypotonia | |
|
Eye disorders |
Eye disorders, including Eye swelling, usually associated with allergic reactions have been reported | |
|
Ear and labyrinth disorders |
Tinnitus | |
|
Cardiac disorders |
Bradycardia, Cyanosis, Tachycardia | |
|
Vascular disorders |
Hypotension, Pallor, Vasodilatation | |
|
Respiratory, thoracic and mediastinal disorders |
Bronchospasm, Dyspnoea, Laryngeal oedema, Pharyngeal oedema and pain, Throat irritation or tightness, Cough. When administered orally, Aspiration, Pneumonia aspiration. | |
|
Gastrointestinal disorders |
Abdominal pain Nausea; Vomiting |
Gastrointestinal signs and symptoms are widely reported, it is not always possible to differentiate between pre-existing medical conditions and procedural complications. Events reported include: Intestinal ischemia Constipation and in severe cases gastrointestinal blockage; Gastrointestinal inflammation, ulceration or perforation; Abdominal discomfort abdominal distension; Diarrhoea; Colitis ulcerative may be aggravated; Retching; Flatulence; Swollen tongue |
|
Skin and subcutaneous tissue disorders |
Skin reactions are varied and most likely to be associated with allergic reactions. Reports include: Erythema, Dermatitis Contact, Excessive granulation tissue, Hyperhidrosis, Periorbital oedema, Pruritus, Rash, |
|
Swelling face, Urticaria | ||
|
Renal and urinary disorders |
Dysuria | |
|
General disorders and administration site conditions |
Malaise, Pain, Swelling, Asthenia, Pyrexia, Face oedema | |
|
Investigations |
Electrocardiogram abnormal | |
|
Injury and poisoning |
Intravasation by barium sulfate, associated with pre-existing bowel disease or diverticulitis, has been reported rarely. Barium impaction |
More rarely and depending on the route of administration, i.e. oral or rectal, the following procedural complications have been reported:
Infections (e.g. peritonitis) subsequent to existing or new gastrointestinal perforation. Complications include adhesions and granuloma.
Subsequent to existing or procedural gastrointestinal trauma, intravasation of barium sulfate with rare subsequent venous emboli formation, including the hepatic portal vein, vena cava and pulmonary embolism that may be fatal in approx 50% of cases.
Please see section 4.4 for measures to be taken to avoid these adverse reactions, and actions to take if such adverse reactions occur.
Very rare cases of death associated with barium sulfate administration have been reported in the literature. The majority of the deaths relate to procedural complications usually caused by failure to follow generally accepted radiological practice. Some cases had a history indicating that barium sulfate administration was highly unlikely to be a primary or even secondary causative factor in patient fatality.
Paediatric patients
The type of adverse reactions is similar in children and adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme - Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
Barium sulfate is non-toxic and absorbed systemically in negligible amounts.
Repeated use within a very short period of time has led to abdominal cramps, nausea, vomiting, diarrhoea, and constipation. These symptoms are transitory in nature and may be allowed to resolve without medical intervention or may be treated according to currently accepted standards of care.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: X-ray contrast media, barium sulfate with suspending agents, ATC code: V08BA01
The active constituent of Polibar, barium sulfate, is inert and has no pharmacodynamic properties. It serves only as a radiopaque substance to opacify the gastro-intestinal tract during X-ray examinations.
5.2 Pharmacokinetic properties
Under physiological conditions, barium sulfate passes through the gastrointestinal tract in an unchanged form and is absorbed only in small, pharmacologically insignificant amounts.
5.3 Preclinical safety data
There are no preclinical data of relevance to the prescriber which are additional to that already included elsewhere in the SPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Gum ghatti Sorbitol (E420)
Sodium citrate (E331)
Sodium carrageenan (E407)
Simeticone
Polyoxyethylene glyceryl mono-oleate Citric acid anhydrous (E330)
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
Three years.
This pack is for single-use only. Polibar should be administered immediately following reconstitution and must not be stored.
6.4 Special precautions for storage
Store below 25 °C. Store in the original package.
For storage conditions after reconstitution of the medicinal product, see section 6.3
6.5 Nature and contents of container
Unit dose prefilled enema bag composed of polyvinylchloride containing 397 g, 567 g or 680 g of product. Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling
Adults: Polibar should be suspended over the density range of 20 - 115 %
w/v (20 - 60 % w/w).
Reconstitution information for use of Polibar is provided below.
Attach clamp to tubing and close. Study the appropriate graph below. This shows the density range and how much water to use for each density. Measure the indicated quantity of warm (40 oC) water and add this to the bag through the red snap-cap seal. Hold the bag by the finger holes and shake vigorously for 10-20 seconds. When ready to use shake again, 10-20 seconds. Then with the thumb and forefinger pop the red ball at the bag tube junction into the bag. Run barium through the tubing - attach rectal tube. The kit is now ready.
Water to be added (mL) 397 g enema bag
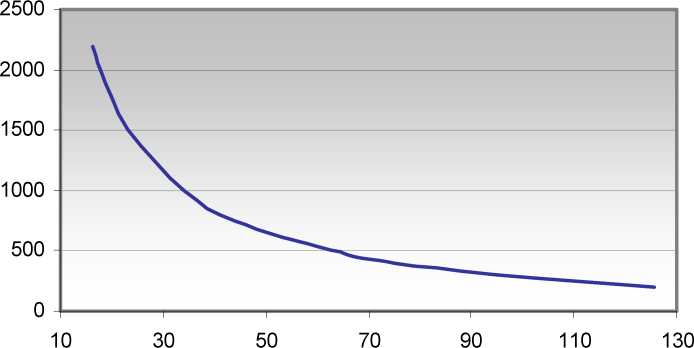
% Barium Sulfate (w/v)
Any unused, medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Bracco Imaging spa Via Egidio Folli 50 20134 Milano Italy
8 MARKETING AUTHORISATION NUMBER(S)
PL 12032/0021
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
7th May 1993 / 15th January 2004
10 DATE OF REVISION OF THE TEXT
26/09/2016