Politrate Depot 22.5 Mg Powder And Solvent For Prolonged-Release Suspension For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Politrate Depot 22.5 mg powder and solvent for prolonged-release suspension for injection.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 22.5 mg of leuprorelin acetate (equivalent to 21.42 mg leuprorelin free base).
1 ml of reconstituted suspension contains 11.25 mg of leuprorelin acetate.
Excipients with known effect:
Each vial contains from 1.6 to 2.7 mg (<1 mmol) of sodium (as carmellose sodium). For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder and solvent for prolonged-release suspension for injection.
Powder: white to off-white powder.
Solvent: clear, colorless and particle free solution (pH 5.0 - 7.0).
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Politrate Depot is indicated for palliative treatment of hormone dependent advanced prostate cancer.
4.2 Posology and method of administration
Posology
The usual recommended dose of Politrate Depot 22.5 mg presented as a three months depot injection and administered as a single intramuscular injection every three months.
Politrate Depot must be administered under direction of healthcare professional having the appropriate expertise for monitoring the response to treatment.
The dose of Politrate Depot 22.5 mg allowing the continuous release of leuprorelin acetate over a three month period is incorporated in a depot formulation. The lyophilized powder should be reconstituted and administered as a single intramuscular injection every three months. Intraarterial or intravenous administration must be avoided. The vial of Politrate Depot microsphere powder should be reconstituted immediately prior to administration by intramuscular injection. As with other drugs administered regularly by injection, the injection site should be varied periodically.
Politrate Depot therapy should not be discontinued when remission or improvement occurs.
Response to Politrate Depot therapy should be monitored measuring serum levels of testosterone as well as prostate-specific antigen (PSA) periodically. Clinical studies have shown that testosterone levels increased during the first 4 days of treatment in the majority of non-orchiectomized patients. They then decreased and reached castrate levels by 3-4 weeks. Once attained, castrate levels (defined as concentration of testosterone equal or less than 0.5 ng/mL) were maintained as long as drug therapy continued.
If a patient's response appears to be sub-optimal, then it would be advisable to confirm that serum testosterone levels have reached or are remaining at castrate levels. Transient increases in acid phosphatase levels sometimes occur early in the treatment period but usually return to normal or near normal values by the 4th week of treatment.
Duration of treatment
Politrate Depot should be administered every three months as intramuscular injections.
As a rule, therapy of advanced prostate cancer with Politrate Depot entails long-term treatment and therapy should not be discontinued when remission or improvement occurs.
Special populations
Pediatric population
The safety and efficacy of Politrate Depot in the paediatric patients has not been established. Therefore, Politrate Depot is not recommended in children or adolescents until safety and efficacy data become available.
Renal/hepatic insufficiency
The pharmacokinetics of Politrate Depot in hepatically and renally impaired patients has not been determined.
Elderly
In the clinical trial for Politrate Depot 22.5 mg, the mean age of the subjects studied was 71.0±9.02 years. Therefore, the labelling reflects the pharmacokinetics, efficacy and safety of Politrate Depot in this population.
Method of Administration
Politrate Depot must be administered via the intramuscular route only. Do not administer by any other route. If it is administered subcutaneously by mistake, the patient should be closely monitored since no data about other administration routes a part from intramuscular is available for Politrate Depot. For instructions on reconstitution of the medicinal product before administration, see section 6.6.
4.3 Contraindications
Hypersensitivity to the active substance, luteinizing hormone releasing hormone (LHRH) analogs or to any of the excipients listed in section 6.1. Reports of anaphylactic reactions to synthetic LHRH or LHRH agonist analogs have been reported in the medical literature.
Previous orchiectomy.
Politrate Depot must not be used as the only treatment in patients with prostate cancer and with evidence of spinal cord compression or spinal metastases.
Politrate Depot is not indicated for use in women.
Politrate Depot is not indicated for use in paediatric patients.
4.4 Special warnings and precautions for use
In the initial stages of Politrate Depot treatment, as occurs during treatment with other LHRH agonists, a transient rise in levels of testosterone may occur. In some cases, this may be associated with a "flare" or exacerbation of the tumor growth resulting in temporary worsening of prostate cancer symptoms. These symptoms usually subside on continuation of therapy (see section 4.8). "Flare" may manifest itself as systemic or neurological symptoms in some cases (i.e. bone pain...). Also, cases of orchiatrophy and gynecomastia have been described with other LHRH agonists.
Treatment should be discontinued immediately if the patient develops any signs or symptoms suggestive of anaphylaxis/anaphylactic reaction (dyspnea, asthma, rhinitis, angioneurotic edema or glottis, hypotension, urticaria, rash, pruritus or interstitial pneumonitis). Patients should be informed before starting treatment, warning them to discontinue it and consult their doctor if any of the above mentioned symptoms occur. Patients who have experienced a hypersensitivity reaction to leuprolide should be closely monitored and should not be rechallenged with Politrate Depot.
In patients treated with leuprorelin acetate, isolated cases of ureteral obstruction (with or without haematuria) and spinal cord compression or metastatic vertebral lesions have been observed, which may contribute to paralysis with or without fatal complications. Patients at risk of ureteral obstruction, spinal cord compression or metastatic vertebral lesions should be considered carefully and closely supervised in the first few weeks of treatment. These patients should be considered for prophylactic treatment with anti-androgens.
Should urological/neurological complications occur, these should be treated by appropriate specific measures.
There is an increased risk of incident depression (which may be severe) in patients undergoing treatment with GnRH agonists, such as leuprolide acetate. Patients should be informed accordingly and treated as appropriate if symptoms occur.
Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with an LHRH agonist. Adding anti-androgenic therapy to the treatment regimen reduces bone loss, but increases the risk of other adverse effects such as clotting problems and edema. If an anti-androgen is used over a prolonged period, due attention should be paid to the contraindications and precautions associated with its extended use. Patients at risk or with a medical history of osteoporosis should be considered carefully and closely supervised during treatment with leuprorelin acetate (see section 4.8).
Hepatic dysfunction and jaundice with elevated liver enzyme levels have been reported with the use of leuprorelin acetate. Therefore, close observation should be made and appropriate measures taken if necessary.
Response to Politrate Depot therapy should be monitored by clinical parameters and by measuring testosterone and PSA serum levels periodically.
Patients may experience metabolic changes (e.g. glucose intolerance or worsening of existing diabetes), hypertension, weight changes, and cardiovascular disorders. As would be expected with this class of drug, development or aggravation of diabetes may occur, therefore diabetic patients may require more frequent monitoring of blood glucose during treatment with Politrate Depot. Patients at high risk for metabolic or cardiovascular diseases should be carefully assessed before commencing treatment and adequately monitored during androgen deprivation therapy. Therapy with leuprorelin acetate results in suppression of the pituitary-gonadal system. Results of diagnostic tests of pituitary gonadotropic and gonadal functions conducted during and after leuprorelin acetate therapy may be affected.
Increased prothrombin time has been reported in patients under treatment with leuprorelin acetate. Leuprolide acetate should be used with caution in patients with known bleeding disorders, thrombocytopenia or on treatment with anticoagulants.
Seizures have been reported with the administration of leuprorelin acetate. These cases were observed in patients with a history of seizures, epilepsy, cerebrovascular disorders, anomalies or central nervous system tumors and in patients with concomitant medications that have been associated with seizures for example bupropion and selective inhibitors of serotonin reuptake (SSRIs). Seizures in patients in the absence of the any medical conditions mentioned above have also been reported.
Leuprolide acetate should be used with caution in the presence of cardiovascular disease (including congestive heart failure condition), thromboembolism, edema, depression, and pituitary apoplexy.
This medicinal product contains less than 1 mmol sodium (23 mg) per vial. It is essentially ‘sodium- free’.
Androgen deprivation therapy may prolong the QT interval.
In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval (see section 4.5) physicians should assess the benefit risk ratio including the potential for Torsade de pointes prior to initiating Politrate Depot.
4.5 Interaction with other medicinal products and other forms of interaction
No pharmacokinetic-based drug-drug interaction studies have been conducted with leuprorelin acetate. However, because leuprorelin acetate is a peptide that is primarily degraded by peptidase and not by Cytochrome P-450 enzymes as noted in specific studies, and the drug is only about 46% bound to plasma proteins, pharmacokinetic drug interactions would not be expected to occur.
Since androgen deprivation treatment may prolong the QT interval, the concomitant use of Politrate Depot with medicinal products known to prolong the QT interval or medicinal products able to induce Torsade de pointes such as class IA (e.g. quinidine, disopyramide) or class III (e.g. amiodarone, sotalol, dofetilide, ibutilide) antiarrhythmic medicinal products, methadone, moxifloxacin, antipsychotics, etc. should be carefully evaluated (see section 4.4).
4.6 Fertility, pregnancy and lactation
Pregnancy:
Politrate Depot is not indicated for use in pregnant women.
Leuprolide acetate injection may cause foetal harm when administered to a pregnant woman. Therefore, spontaneous abortion may occur if the drug is administered during pregnancy.
Breastfeeding:
Politrate Depot should not be used in women who are breastfeeding.
Fertility:
Studies in animals have shown reproductive toxicity (see section 5.3).
4.7 Effects on ability to drive and use machines
No specific studies on the effects of Politrate Depot on the ability to drive and use machines have been performed. However, the ability to drive and use machines may be impaired due to visual disturbances and dizziness.
4.8 Undesirable effects
The safety profile of Politrate Depot is based on the results of a phase III clinical trial performed in patients with prostate cancer treated with two sequential intramuscular doses administered with a 3-month interval of Politrate Depot and followed up for total a period of 6 months. Most of the treatment-related AEs reported are mainly subject to the specific pharmacological action of leuprorelin acetate and associated with testosterone suppressing therapy.
The most commonly reported adverse reactions with Politrate Depot are hot flushes, fatigue, asthenia, hyperhidrolisis, nausea and bone pain.
The following adverse reactions from clinical investigations were listed below by system organ class and in order of decreasing incidence (very common: >1/10; common: >1/100 to <1/10; uncommon: > 1/1,000 to < 1/100; rare: >1/10,000 to <1/1,000; very rare: < 1/10,000).
Table 1. Number and frequency of ADRs during Politrate Depot 22.5 mg therapy.
Category
SOC
Frequency: PT
Metabolism and nutrition disorders:
Common: decreased appetite
Uncommon: hypercholesterolaemia_
Psychiatric disorders
Common: Insomnia, libido decreased. Long term use: mood changes, depression.
Uncommon: Sleep disorders, emotional disorder, anxiety, anger. Short term use:
_mood changes, depression._
Nervous system disorders
Common: Dizziness
Uncommon: dysgeusia, formication, headache, lethargy_
Eye disorders
Uncommon: vision blurred_
Respiratory, thoracic and mediastinal disorders
Uncommon: Pleurisy
Not known:_Pneumonitis, interstitial lung disease_
Ear and labyrinth disorders
Uncommon: Tinnitus_
Vascular disorder
Very Common: Hot flush
Common:_flushing_
Gastrointestinal disorders
Common: Nausea, diarrhoea
Uncommon: Abdominal pain upper, constipation_
Skin and subcutaneous tissue disorders
Common: Hyperhidrosis, pruritus , cold sweats
Uncommon: Papule, rash, pruritus generalised, , night sweats_
Muskuloeskeletal and connective tissue disorders
Common: Bone pain, arthralgia
Uncommon: Back pain, musculoskeletal pain, neck pain_
Renal and urinary disorders
Common:_Pollakiuria, nocturia, urinay tract pain, urine flow decreased_
Reproductive system and breast disorders
Common: Erectile dysfunction
Uncommon: Nipple pain, pelvic pain, testicular atrophy, testicular disorder_
General disorders and administration site conditions
Common: Fatigue, asthenia, pain, local adverse reactions (see table 2)
Uncommon: Feeling hot, hyperhidrosis_
Investigations
Common: Alanine aminotrasferase Increased, aspartate aminotransferase
increased, blood triglyceride increased, blood creatine phosphokinase increased, blood glucose increased
Uncommon: Blood calcium increased, blood creatine increased, blood lactate
dehydrogenate increased, blood potassium decreased, blood potassium increased, blood urea increased, electrocardiogram QT prolonged (see sections 4.4 and 4.5), electrocardiogram QT shortened, _electrocardiogram T wave inversion, gamma-glutamyltransferase
increased, glomerular filtration rate decreased, haematocrit decreased, haematology test abnormal, haemoglobin decreased, mean cell volume increased, red blood cell count decreased, residual urine volume increased
In terms of severity, 84.7% of all treatment-related AEs were mild or moderate. The most frequently reported was hot flushes (77.3%), 57.7% of the hot flushes were reported as mild and 17.2% as moderate. Five cases of hot flushes (3.1%) were reported as severe.
A total of 38 local adverse reactions (LAR) at the injection site were reported by 24 patients (14.7%) during the study.
Local adverse reactions reported after the injection of after Politrate Depot 22.5 mg are similar to the local adverse reactions associated with similar products administered via intramuscular. Injection site pain, injection site erythema and injection site induration were the most commonly reported. Uncommonly reported reactions were injection site discomfort, injection site urticaria, injection site warmth, vessel puncture site pain, arthralgia, musculoskeletal pain and injection site hemorrhage (Table 2)
Table 2. Frequency of patients with local adverse reactions during Politrate Depot therapy.
|
Primary SOC* |
Patients with related LAR |
|
PT: General disorders and administration site conditions |
% |
|
Very common | |
|
Injection site pain |
10.4 |
|
Common | |
|
Injection site erythema |
3.1 |
|
Injection site induration |
2.5 |
|
Uncommon | |
|
Injection site discomfort |
0.6 |
|
Injection site urticaria |
0.6 |
|
Injection site warmth |
0.6 |
|
Injection site |
0.6 |
|
hemorrhage | |
|
Arthralgia |
0.6 |
|
Musculoskeletal pain |
0.6 |
|
Vessel puncture site |
0.6 |
|
pain | |
*Subjects may fall into more than one category; LAR: local adverse reaction; SOC: System Organ Class.
These events were all reported as not serious and mild or moderate in severity. No patient discontinued therapy due to local adverse events.
Other adverse events which have been reported in general to occur with leuprorelin acetate treatment include:
Peripheral oedema, pulmonary embolism, palpitations, myalgia, muscle weakness, chills, peripheral vertigo, rash, amnesia, visual disturbances and an alteration in the skin sensation. Infarction of pre-existing pituitary apoplexy has been reported rarely after administration of both short and long acting LHRH agonists. There have been rare reports of thrombocytopenia and leucopenia. Changes in glucose tolerance have been reported.
Changes in Bone Density
Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with LHRH analogues. It can be anticipated that long periods of treatment with leuprorelin may show increasing signs of osteoporosis. Regarding the increased risk for fractures owing to osteoporosis (see section 4.4).
Exacerbation of signs and symptoms of the disease
Treatment with leuprorelin acetate can cause exacerbations of signs and symptoms of the disease during the first few weeks. If conditions such as vertebral metastases and/or urinary obstruction or haematuria are aggravated, neurological problems such as weakness and/or paraesthesia of the lower limbs or worsening of urinary symptoms may occur (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
4.9 Overdose
There is no clinical experience with the effects of an acute overdose of Politrate Depot or leuprorelin acetate. In clinical trials using daily subcutaneous leuprorelin acetate in patients with prostate cancer, doses as high as 20 mg/day for up to two years caused no AEs differing from those observed with the 1 mg/day dose.
In animal studies, doses of up to 500 times the recommended human dose resulted in dyspnea, decreased activity and local irritation at the injection site. In cases of overdosage, the patient should be monitored closely and management should be symptomatic and supportive.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Endocrine therapy. Hormones and related agents. Gonadotropinreleasing hormones analogues; ATC code: L02AE02.
The chemical name of Leuprorelin acetate is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-L-prolyl-ethylamide.
Leuprorelin acetate is inactive when given orally due to poor membrane permeability and an almost complete inactivation by intestinal proteolytic enzymes.
Leuprorelin acetate has potent LHRH agonist properties when given during short-term and intermittent therapy, however, when administered in a continuous, nonpulsatile manner, LHRH analogs induce inhibition of gonadotropin secretion and suppression of testicular steroidogenesis.
Upon binding to pituitary LHRH receptors, leuprorelin acetate produces an initial increase in circulating levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH), leading to an acute rise in levels of testosterone and dihydrotestosterone. However, within five to eight days after drug administration, LHRH analogs produce de sensitization of the LHRH receptor complex and/or downregulation of the anterior pituitary gland. Due to the fact that there are fewer receptors on the cell surface, cellular stimulation is decreased, and less gonadotropin is synthesized and secreted. Eventually, after several weeks of LHRH agonist therapy, LH and FSH secretion is suppressed. As a result, Leydig cells in the testes cease to produce testosterone, and the serum testosterone concentration declines to a castration level (less than 0.5 ng/mL) in about two to four weeks after initiation of treatment.
In an open-label, multicenter, multiple dose clinical study of Politrate Depot 22.5 mg, 163 patients with prostate cancer, were enrolled. The objectives were to determine the efficacy and safety of Politrate Depot when given to prostate cancer patients who could benefit from androgen deprivation therapy. Politrate Depot was administered intramuscularly in 2 doses with a 3-month interval.
Testosterone levels were monitored at different days during 168 days. Testosterone sampling schedule was at days 0 (1 and 4 hrs), 2, 14, 28, 56, 84 pre-dose, 84 (1h and 4 hrs), 86, 112 and 168. Primary end point was defined as testosterone values < 0.5 ng/mL and no missing data assessed at days 28, 84 and 168. For each patient, if testosterone was greater than 0.5 ng/mL or if testosterone data were missing at any of the key time points (ie, Days 28, 84, and 168), the patient was classified as a failure, unless the missing data were due to an event, such as death, unrelated to study drug. Specifically, if at any key time point (Days 28, 84, and 168) missing data were due to an AE related to study drug or treatment, the patient was classified as a failure.
After the first injection the mean testosterone levels rapidly increased from baseline levels (4.09±1.79 ng/mL), reaching peak levels (Cmax) of 6.33±3.40 ng/mL at the second day. After peaking, testosterone levels fell, and 98.8% (159/161) of the evaluable patients achieved medical castration at day 28 (defined as testosterone less than 0.5 ng/mL). Additionally, at this time, 77.0% of patients achieved the more stringent criterion of testosterone < 0.2 ng/mL. (Figure 1). At day 168, 99.4% of evaluable patients (150/151) presented testosterone level below 0.5 ng/mL and 90.7% were below < 0.2 ng/mL.
According to the primary endpoint definition (see definition above) the rate of patients maintaining castration throughout the study was 98.1% (158/161).
Figure 1. Mean (±SD) testosterone plasma levels during two sequential IM doses of
Politrate Depot 22.5 mg with a 3-month interval
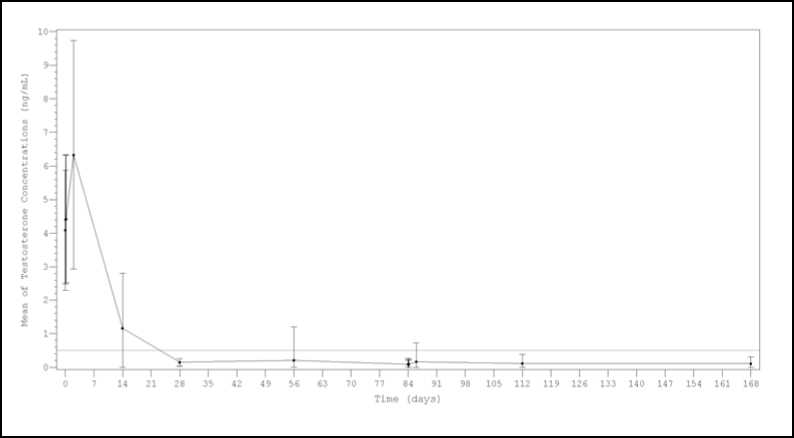
Results from a sensitivity analysis performed considering either single testosterone escapes or missing data as failures, showed castration rates around or above 92% at every time point ( Day 28, 97.5% (157/161); Day 56, 93.2% (150/161); Day 84predose, 96.9% (156/161); Day 84]hour post-dose 91.9% (148/161); Day 84^ post-dose 91.9% (148/161); Day 86 93.8% (151/161); Day 112 92.5% (149/161) and Day 168 93.2% (150/161)).
The frequency of escapes just after the second administration was 6.8% (11/161) and the frequency of testosterone breakthrough response was 6.2% (10/161). None of the transient escapes was associated with LH increase, clinical symptoms or PSA raises.
No drug-related adverse events suggestive of a clinical testosterone flare (urinary retention, spinal cord compression, or exacerbation of bone pain) were reported in any of the patients showing a testosterone breakthrough effect.
Secondary efficacy endpoints included determination of serum LH, FSH and PSA concentrations. By day 14 after the first Politrate Depot injection, mean LH and FSH serum levels had decreased below the baseline concentrations. Concentrations remained well below baseline values from day 28 until the end of the study. During the treatment, median PSA serum levels gradually decreased (first month) and then remained constantly below baseline level until the end of the study. However, as expected a wide and expected inter-individual variation in PSA concentrations was observed throughout the study.
5.2 Pharmacokinetic properties
Absorption
Following two sequential injections of Politrate Depot administered with a 3-month interval, maximal leuprorelin acetate plasma concentration observed in a sample of prostate cancer patients (N=30) was similar among the three cycles. After first administration (Days 0-84), Cmax was 46.79±18.008 ng/mL. Mean time to achieve Cmax (Tmax) was 0.07 days, corresponding to 1.68 h (range 1.008 - 4.008 h).
Distribution
No drug distribution study was conducted with Politrate Depot. However, in healthy male volunteers, the mean steady-state volume of distribution of leuprorelin acetate following bolus intravenous (IV) 1.0 mg dose was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Elimination
No drug metabolism or excretion studies were conducted with Politrate Depot.
Leuprorelin is expected to be metabolised to smaller inactive peptides that may be excreted or further catabolised.
In healthy male volunteers, a 1.0 mg bolus of leuprorelin acetate administered IV revealed that the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model.
Following administration of leuprorelin acetate to 3 patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
Special Populations
Renal/hepatic insufficiency
The pharmacokinetics of the drug in hepatically and renally impaired patients have not been determined.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity and genotoxicity conducted with leuprorelin acetate.
As expected from its known pharmacological properties, non-clinical studies showed effects on the reproductive systems, which were reversible. In the reproductive toxicity studies, leuprorelin acetate did not show teratogenicity. However, embryotoxicity/lethality was observed in rabbits.
Carcinogenicity studies performed in rats with leuprorelin acetate administered subcutaneously (0.6 to 4 mg/kg/day), showed a dose-related increase in pituitary adenomas. Furthermore a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males was observed the highest incidence was in the low dose group. Administration of leuprorelin acetate resulted in inhibition of the growth of certain hormone dependent tumors (prostatic tumors in Noble and Dunning male rats and DMBA-induced mammary tumors in female rats). No such effects were observed in carcinogenicity studies performed in mice. No carcinogenicity studies have been conducted with Politrate Depot.
Studies with leuprorelin acetate showed that the product was not mutagenic in a set of in vitro and in vivo assays. No mutagenicity studies have been conducted with Politrate Depot.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Excipients of the lyophilizate (vial):
Polysorbate 80 Mannitol (E-421)
Carmellose sodium (E-466)
Triethyl citrate Poly(lactic acid) (PLA)
Excipients of the solvent (prefilled syringe):
Mannitol (E-421)
Sodium hydroxide (for pH adjustment) Hydrochloric acid (for pH adjustment) Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
No other solvent other than the sterile solvent provided for Politrate Depot can be used for the reconstitution of Politrate Depot powder.
6.3 Shelf life
3 years unopened.
Once reconstituted with the solvent the suspension obtained should be administered immediately.
6.4 Special precautions for storage
Do not store above 25o C. Do not freeze.
6.5 Nature and contents of container
The commercial kit includes:
1.One (1) type I glass vial containing 22.5 mg of leuprorelin acetate as a freeze-dried powder, sealed with an elastomeric stopper and a aluminium cap with plastic flip-off.
2.One (1) type I glass prefilled syringe containing 2 mL of solvent sealed with an elastomeric cap.
3.One (1) polycarbonate / HDPE adaptor system including one (1) sterile 20 gauge needle.
6.6 Special precautions for disposal
Method of administration
The vial of Politrate Depot microsphere powder should be reconstituted immediately prior to administration by intramuscular injection. Make sure an aseptic technique is followed.
The reconstituted product is a suspension of milky, white colour appearance.
No other solvent can be used for reconstitution of Politrate Depot.
Reconstitute Politrate Depot according to the following instructions:
1
I
Remove cap from the vial
2

Attach the adaptor system (in purple) to vial until a ‘clicking’ sound is heard
5
3
Affix the white finger-grip to the diluents-containing
syringe
Remove the rubber cap from the syringe and attach it to the adaptor system
6
4
While keeping the syringe and vial securely coupled in an upright position, slowly push the plunger in order to transfer all the diluents into the vial
7
With the syringe still
coupled to the vial, shake
the vial gently for
approximately one minute until a uniform milky-white suspension is
obtained

Detach the syringe and needle from the adaptor system by twisting the upper piece of the adaptor counter-clockwise. The drug is ready to be used.

Turn the system upside down, and carefully pull out the plunger to draw up the re-suspended drug from the vial into the syringe
8

Clean the injection area with an alcohol swab and let the skin dry.
Inject the suspension intramuscularly into the upper outer quadrant of the gluteus
Some product may cake or clump at the vial wall. This is considered normal. During product manufacture the vial is filled with excess product in order to make sure that a final dose of 22.5 mg of leuprorelin acetate is administered.
The product is meant for a single injection. Any remaining suspension must be discarded.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
GP-PHARM, S.A.
Pol ind Els Vinyets -els Fogars. Sector 2 Carretera comarcal 244, km22 08777 Sant Quinti de Mediona.
Spain
8 MARKETING AUTHORISATION NUMBER(S)
PL 25859/0005
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
22/05/2015
10 DATE OF REVISION OF THE TEXT
06/10/2015