Politrate Depot 3.75 Mg Powder And Solvent For Prolonged-Release Suspension For Injection
Politrate Depot 3.75 mg

Module 1 Section 1.3 Page 1/11
Package leaflet: Information for the user
Politrate Depot 3.75 mg powder and solvent for prolonged-release suspension for
injection
leuprorelin acetate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What Politrate Depot is and what it is used for
2. What you need to know before you use Politrate Depot
3. How to use Politrate Depot
4. Possible side effects
5. How to store Politrate Depot
6. Contents of the pack and other information
1. What Politrate Depot is and what it is used for
Politrate Depot is a vial containing a white powder, which is made into a suspension for injection into a muscle. Politrate Depot contains the active ingredient leuprorelin (also called leuprolide), which belongs to a group of medicines called luteinizing hormone releasing hormone (LHRH) agonists (medicines that reduce testosterone - a sex hormone).
Your doctor has prescribed Politrate Depot for palliative treatment of advanced prostate cancer.

Module 1 Section 1.3 Page 2/11
2. What you need to know before you use Politrate Depot
Do not use Politrate Depot:
- if you are allergic (hypersensitive) to LHRH, LHRH agonists or any of the other ingredients of this medicine (listed in section 6). An allergic reaction may include rash, itching, difficulty of breathing or swelling of the face, lips, throat or tongue.
- if you have had an orchiectomy (removal of the testicles).
- if you are female or a child.
- Politrate Depot must not be used alone for the treatment of prostate cancer when the spinal cord is compressed or the cancer has spread to the spine.
Warnings and precautions
• Talk to your doctor or pharmacist before you are given Politrate Depot
• Please tell your doctor if you have any of the following:
Any heart or blood vessel conditions, including heart rhythm problems (arrhythmia), or are being treated with medicines for these conditions. The risk of heart rhythm problems may be increased when using Politrate Depot.
• Your condition may get worse at first during the first weeks of the treatment, but should improve with continued treatment. Such signs and symptoms include: temporary increase of testosterone (a male hormone), hot flushes, bone pain, nervous system disorders (including depression) or urinary obstruction.
• If you feel you have experienced an allergic reaction (shortness of breath, asthma, rhinitis, swelling of the face, urticaria, skin eruption), stop using this medicine and inform your doctor.
• Tell your doctor if you might be at risk or if you have any of the following as you may need more frequent check ups:
• you suffer from any unexplained bruising or bleeding or if you feel generally unwell. Although rare, these could be symptoms of changes in the number of red or white cells
• you have metabolic disease

Module 1 Section 1.3 Page 3/11
• you have heart problems, or a pounding heart beat
• you have diabetes
• Your doctor should be aware of any previous personal clinical history of pituitary adenoma (non-cancerous tumor of pituitary). Cases of pituitary apoplexy (partial tissue loss of pituitary gland) have been described after initial administration of this type of drug to patients with pituitary adenoma. Pituitary apoplexy may be manifested by sudden headache, meningismus, visual disturbances or altered vision, even blindness, and occasionally, decrease in the level of consciousness.
• Your doctor should be aware if you suffer from a bleeding disorder, thrombocytopenia or if you are on treatment with anticoagulants. Your liver function may need to be monitored as changes to the liver and jaundice (yellow eyes and skin) have been reported with leuprorelin treatment.
• A fractured spine, paralysis, low blood pressure and high blood pressure have been reported with leuprorelin treatment.
• There have been reports of depression in patients taking Politrate Depot which may be severe. If you are taking Politrate Depot and develop depressed mood, inform your doctor.
• A decreased bone density (brittleness or thinning of the bones) has been reported with leuproreline. Your doctor may consider adding an antiandrogen to the treatment with Politrate Depot. Your doctor will be on the alert for inflamed veins (thrombophlebitis) and other signs of clotting disorders and oedema (swelling of hands, feet or ankles). There is an increased risk of these occurring in the case that an antiandrogen treatment is added to Politrate Depot.
• Tell your doctor, if you feel pressure on the spinal cord and/or experience urinary disorders and/or haematuria (blood in the urine). In this case your doctor will be take if necessary, additional precautions to avoid neurological complications (e.g. tingling in hands and feet, paralysis) or obstruction of the urethra (the tube that connects the bladder to the outside of the body). You will be closely supervised during the first weeks of treatment.
• Patients may experience metabolic changes (e.g. glucose intolerance or worsening of existing diabetes), weight changes and cardiovascular disorders.

Module 1 Section 1.3 Page 4/11
• Patients with metabolic or cardiovascular disease and especially patients with history of congestive heart failure (condition in which the heart can no longer pump enough blood to the rest of the body) should be monitored during treatment with leuprorelin.
• You will need some blood tests during treatment, to check that Politrate Depot is being efficacious.
• You may experience a loss of interest in sexual intercourse, hot flushes and occasionally there may be a reduction in size and function of the testes.
• You may become fertile again when Politrate Depot treatment is stopped.
• Politrate Depot may interfere with certain laboratory tests so make sure your doctor knows you are using Politrate Depot
• Politrate Depot contains an ingredient which may give a positive test result in doping controls
• Convulsions can occur in predisposed patients (those with a history of seizures, epilepsy, cerebrovascular disorders, anomalies or central nervous system tumors), in patients receiving drugs that can cause seizures and, to a lesser extent, in other patients who do not have these characteristics.
Other medicines and Politrate Depot
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. It may still be all right for you to be given Politrate Depot and your doctor will be able to decide what is suitable for you.
Politrate Depot might interfere with some medicines used to treat heart rhythm problems (e.g.quinidine, procainamide, amiodarone and sotalol) or might increase the risk of heart rhythm problems when used with some other drugs(e.g. methadone (used for pain relief and part of drug addiction detoxification), moxifloxacin (an antibiotic), antipsychotics used for serious mental illnesses).
Pregnancy and breast-feeding
Politrate Depot is not indicated for use in women.
This medicine is contraindicated during pregnancy. Spontaneous abortions may occur if this medicine is administered during pregnancy.

Module 1 Section 1.3 Page 5/11
Driving and using machines
Disturbance of vision and dizziness can occur during treatment. If affected you should not drive or operate machinery.
Politrate Depot contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium- free’.
3. How to use Politrate Depot
Dose
Politrate Depot must be given under the supervision of a doctor or a qualified health practitioner.
Adults including the elderly:
The recommended dose of Politrate Depot is an injection once a month. The powder is made up into a suspension and given as a single injection intramuscularly (into a muscle) once a month (approximately every 28 to 33 days).
The injection site should be varied at regular intervals.
Politrate Depot must be administered via the intramuscular route only. Do not administer by another route.
Use in children: Politrate Depot is not indicated for use in children..
The strength of your treatment is decided by your doctor.
If you use more Politrate Depot than you should
This is unlikely as your doctor or nurse will not know the correct dosage. However, if you suspect you have received more than you should, let your doctor know about it immediately so appropriate measures can be taken.
If you forget a dose of Politrate Depot
It is important not to miss a dose of Politrate Depot. As soon as you realise you have missed an injection contact your doctor who will be able to give you your next injection.

Module 1 Section 1.3 Page 6/11
If you stop using Politrate Depot
Since the medical treatment involves administration of Politrate Depot for a long period, when the treatment is interrupted you may experience a worsening of the symptoms related to the disease. Therefore you must not interrupt the treatment prematurely without your doctor’s permission.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Tell your doctor straight away if you get any sudden wheeziness, difficulty in breathing, swelling of the eyelids, face or lips, rash or itching (especially affecting your whole body).
The following side effects have been reported:
Very common (These may affect more than 1 in 10 people):
Hot flushes and injection site reactions.
Common (These may affect up to 1 in 10 people):
Night sweats, cold sweats, fatigue, headache, pyrexia (rise in body temperature), increased appetite, erectile dysfunction, hyperhidrosis (increased sweating), asthenia (lack or loss of strength), back pain and injection site reactions such as pain, irritation, discomfort, erythema (redness of the skin), swelling (increment in size or inflation) bruising (contusion), mood changes and depression in long term use of leuprorelin.
Uncommon (These may affect up to 1 in 100 people):
Breast swelling, breast tenderness, spinning sensation (vertigo), weakness, sleep disorders, somnolence (sleepiness), insomnia ( not sleeping), low tummy pain, diarrhea, feeling sick (nausea), vomiting, feeling hot and cold, feeling jittery, fever, yellow eyes and skin (jaundice), changes in liver enzymes, anorexia (not eating), high cholesterol, joint pain,

Module 1 Section 1.3 Page 7/11
muscle spasms, pain in the hands and feet, decreased sex drive, mood alterations, urine retention, frequent need to urinate, uncontrolled urine (incontinence), swelling around the eyes, ejaculation failure, hyperlipidemia (high levels of blood lipids, pruritus (itching), urticaria (nettle rash), mood changes depression in short term use of leuprorelin, and injection site reactions such as: swelling, injury and hemorrhage.
Not known:
Cardiac disorders: Changes in ECG (QT prolongation).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. You can also report side effects directly via the national
reporting system:
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Politrate Depot
Your doctor or pharmacist will know how to store Politrate Depot.
Keep this medicine out of sight and reach of children.
Do not store above 25o C. Do not freeze.
Store in the original package, in order to protect from light
Do not use this medicine after the expiry date as indicated on the box, vial and pre-filled syringe after "EXP". The syringe has the same expiry date to that of the vial. The expiry date refers to the last day of that month.
Once reconstituted with the solvent the suspension should be administered immediately.

Module 1 Section 1.3 Page 8/11
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Politrate Depot contains
The active substance is leuprorelin acetate. Each vial contains 3.75 mg of leuprorelin acetate. The other ingredients are: Polysorbate 80, Mannitol (E-421), Carmellose sodium (E-466), Triethyl citrate and Poly(DL-lactide-co-glycolide) (PLGA).
The solvent contains (pre-filled syringe): mannitol, water for injection, sodium hydroxide (for pH adjustment) and hydrochloric acid (for pH adjustment).
The concentration of the reconstituted product is 1.8 75 mg/ml.
What Politrate Depot looks like and contents of the pack
Each pack contains a vial with 3.75 mg of leuprorelin acetate, one prefilled syringe with 2 ml of solvent, one adaptor system and one sterile 20 gauge needle.
Marketing Authorisation Holder and Manufacturer GP-PHARM, S.A.
Pol ind Els Vinyets -els Fogars. Sector 2 Carretera comarcal 244, km22 08777 Sant Quinti de Mediona.
Spain
This medicinal product is authorised in the Member States of the EEA under the following names:
Spain: Lutrate Depot 3.75 mg polvo y disolvente para suspension de liberation prolongada inyectable
Germany: Lutrate Depot 3.75 mg Pulver und Losungsmittel zur Herstellung einer Depot-Injektionssuspension
Portugal: Lutrate Depot 3.75 mg / 2 ml po e veiculo para suspensao injectavel de liberta9&o prolongada

Module 1 Section 1.3 Page 9/11
Greece: Lutrate Depot 3.75 mg Kovt^ Kai Siakbrn^ yta napaoKsup svso^pou svatropppaTO<; napaxsTapevn^ anoSsopsuop^
Italy: Politrate
Sweden: Politrate 3.75 mg pulver och vatska till injektionvaska, suspension Hungary: Politrate Depot 3.75 mg Denmark: Lutrate Depot Finland: Lutrate 3.75 mg
Ireland: Leuprorelin 1-month Depot 3.75 mg powder and solvent for prolonged-release suspension for injection
United Kingdom: Politrate Depot 3.75 mg powder and solvent for prolonged-release suspension for injection
Belgium: Lutrate Depot 3.75 mg poeder en oplosmiddel voor suspensie voor injectie met verlengde afgifte
The Netherlands: Leuproreline Lutrate Depot 3.75 mg poeder en oplosmiddel voor suspensie voor injectie met verlengde afgifte Norway: Lutrate Depot
Austria: Lutrate Depot 3.75 mg Pulver und Losungsmittel zur Herstellung einer Depot-
Injektionssuspension
Estonia: Lutrate Depot 3.75 mg
Lithuania: Lutrate Depot 3.75 mg milteliai ir tirpiklis pailginto atpalaidavimo injekcinei Latvia: Lutrate Depot 3.75 mg pulveris un skidinatajs ilgstosas darbibas injekciju suspensijas pagatavosanai
Czech Republic: Lutrate Depot 3.75 mg
Poland: Lutrate Depot
Slovak Republic: Lutrate Depot 3.75 mg
Romania: Lutrate Depot 3.75 mg pulbere §i solvent pentru suspensie injectabila cu eliberare prelungita
Bulgaria: Lutrate Depot
This leaflet was last revised in May 2016.
The following information is intended for healthcare professionals only:

Module 1 Section 1.3 Page 10/11
How to prepare the injection?
Follow these instructions carefully.
An aseptic technique should be observed during the reconstitution procedure.
Important:
Allow the product to reach room temperature before administration. Once mixed, the product must be administered immediately.
This product is for single use only.
Verify the contents of the kit and make sure it includes everything that’s mentioned in the leaflet.
The pack contains:
1 (one) vial of Politrate Depot 3.75 mg (leuprorelin acetate) powder for suspension for injection
1 (one) pre-filled syringe containing the suspension solvent (mannitol 0.8% solution for injection);
1 (one) device for reconstitution;
1 (one) single-use sterile needle.

Module 1 Section 1.3 Page 11/11
1

Remove blue cap from the vial
4
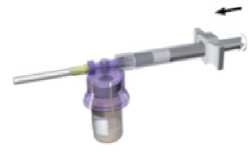
While keeping the syringe and vial securely coupled in an upright position, slowly push the plunger in order to transfer all the diluent into the vial
7
I
2

Attach the adaptor system (in purple) to vial until a 'clicking' sound is heard
5

With the syringe still coupled to the vial, shake the vial gently for approximately one minute until a uniform milky-white suspension is obtained
8
3
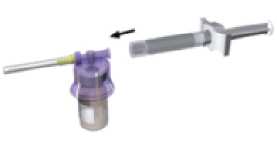
Affix the white finger-grip to the diluents-containing syringe. Remove the rubber cap from the syringe and attach it to the adaptor system
6

Turn the system upside down, and carefully pull out the plunger to draw-up the resuspended drug from the vial into the syringe


Detach the syringe and needle from the adaptor system by twisting the upper piece of the adaptor counter-clockwise. The drug is ready to be used.
Clean the injection area with an alcohol swab and let the skin dry. Inject the suspension
intramuscularly into the upper outer quadrant of the gluteus
Uk-pl-v23-renewalresponses-clean