Pollenase Allergy Relief 50 Micrograms / Dose Nasal Spray

PATIENT LEAFLET: INFORMATION FOR THE USER
Hayfever Relief 50 micrograms Nasal Spray
Contains 50 micrograms of Beclometasone Dipropionate per spray
The name of your medicine is Hayfever Relief 50 micrograms Nasal Spray, which will be referred to as Hayfever Relief Spray throughout the rest of this document.
Read all of this leaflet carefully before you start taking this medicine.
• This medicine is available without prescription. However, you still need to use Hayfever Relief Spray carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• If any of the side effects gets serious or if you notice any side effects not listed in the leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Hayfever Relief Spray is and what it is used for
2. Before you use Hayfever Relief Spray
3. How to use Hayfever Relief Spray
4. Possible side effects
5. How to store Hayfever Relief Spray
6. Further information
1. WHAT HAYFEVER RELIEF SPRAY IS AND WHAT IT IS USED FOR
Beclometasone Dipropionate is a steroid. It is used for the treatment and prevention of allergic rhinitis, including hayfever. It acts by reducing the inflammation of your nose which may be the cause of your sneezing, or an itchy, blocked or runny nose.
2. BEFORE YOU USE HAYFEVER RELIEF SPRAY
Do not use Hayfever Relief Spray if you:
• are allergic (hypersensitive) to Beclometasone Dipropionate or any of the other ingredients of Hayfever Relief Spray (see Section 6 for all the ingredients of this medicine).
• are under 18 years of age. Children or adolescents under 18 years should discuss their hayfever treatment with a pharmacist or doctor.
Take special care and talk to your doctor or pharmacist before using this nasal spray if you:
• have kidney problems
• have ever suffered from glaucoma (increased pressure in the eye, eye pain or blurred vision)
• are suffering from either a nose infection or sinus trouble
• have recently had surgery or an injury to your nose or ulcers in your nose
• are taking or have recently been treated with other corticosteroid medicines such as asthma inhalers, tablets, injections, nasal sprays, eye or nose drops, creams or ointments.
• are taking any other medicines
If you develop a rash or swelling, stop taking this medicine and tell your doctor immediately.
Pregnancy and breast-feeding If you are pregnant, think you might be pregnant, intend to become pregnant, or are breast-feeding, speak with your doctor before using this medicine. Ask your doctor or pharmacist for advice before using any medicine.
3. HOW TO USE HAYFEVER RELIEF SPRAY
Always use Hayfever Relief Spray exactly as described in the leaflet.
Using this medicine (Adults aged 18 or over)
Hayfever Relief Spray is not to be used by children or adolescents under 18 years of age.
The recommended dose is two sprays into each nostril twice a day. The maximum daily dose is a total of eight sprays.
Use your nasal spray regularly for maximum relief. It may take a few days for your medicine to work.
Do not exceed the dose stated in this leaflet.
Once your symptoms have improved, you may be able to reduce to one spray into each nostril twice a day. However, if you notice that your symptoms become worse you should increase the daily dose to two sprays into each nostril twice a day. If your symptoms have not improved after 14 days consult your doctor or pharmacist.
Do not use this product continuously for more than 3 months without consulting your doctor or pharmacist.
If you are suffering from an allergy which also causes watery or irritated eyes, you may need to consult your doctor or pharmacist for some extra medication.
Method of administration
If you have any problems about using your nasal spray, ask your pharmacist.
1. Remove the dust-cap. Shake the bottle gently.
2. On first using the nasal spray prepare for use by pressing down on the white collar using both your index and middle fingers. Keep the base supported with your thumb (figure 2).
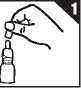
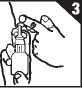
Continue to press down until the collar stops and then allow it to return to its original position. Repeat this action until a fine spray appears. First five attempts to produce a spray should be allowed to go to waste. Now the spray is ready to use.
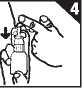
3. To use the spray, first blow your nose gently. Closing one nostril off as shown, bend your head forward slightly. Hold the bottle upright and carefully insert the applicator into the other nostril (figure 3).
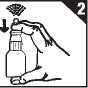
4. Slowly begin to breathe in through your nose and whilst doing so press down firmly on the white collar to produce a fine spray inside your nose. Breathe out through your mouth (figure 4).
5. Repeat step 3 and 4 to squirt a second spray in the same nostril.
Wash the nozzle frequently with warm water. This will prevent it from getting blocked. If the pump has not been used for a short period of time, re-priming may be necessary (see Step 2 above).
If you use more of Hayfever Relief Spray than you should contact your doctor immediately.
If you forget to use Hayfever Relief Spray take a dose as soon as you remember. If it is almost time for your next dose though, do not double the dose, just carry on as before.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS Like all medicines, Hayfever Relief Spray can cause side effects, although not everybody gets them.
If you experience one of the following very rare serious side effects, stop using Hayfever Relief Spray and consult your doctor immediately:
• Swelling of your eyes, face, lips or throat
• You develop a rash
• Shortness of breath or wheezing
• You start feeling faint Other side effects include:
Rare:
• dryness
• irritation of the nose and throat
• unpleasant taste and smell
• nose bleeds
• raised intra-ocular pressure (increased pressure inside the eye which may lead to problems with your eyes such as pain or blurred vision)
• glaucoma (eye disease affecting the optic nerve)
• a hole or perforation in the nasal septum (the skin tissue which separates the nostrils)
In some cases, particularly when Hayfever Relief Spray has been used at high doses for long periods the normal production of steroids in the body is affected. This is more likely to happen if you use high doses for a long time. Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE HAYFEVER RELIEF SPRAY Keep out of the reach and sight of children
Do not use Hayfever Relief Spray after the expiry date which is stated on the label and carton. The expiry refers to the last day of that month.
Discard the contents 3 months after first opening.
• Store below 25°C
• Do not refrigerate
• Protect from light
Medicines should not be disposed of via wastewater or household waste. Ask your Pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Hayfever Relief Spray contains
The active substance is Beclometasone Dipropionate and each spray contains 50 micrograms.
The other ingredients are dextrose anhydrous, polysorbate 80, dispersible cellulose, benzalkonium chloride 95%, phenylethanol and purified water.
Hayfever Relief Spray is supplied in a white plastic (high density polyethylene) bottle fitted with a screw on pump covered by a dustcap. The bottle provides 200 sprays.
Marketing Authorisation Holder and Manufacturer
Manufactured for The Boots Company PLC Nottingham NG2 3AA by Pharmaserve (North West) Ltd, 9 Arkwright Road,
Astmoor Industrial Estate, Runcorn, Cheshire WA7 1NU. Marketing Authorisation is held by Ayrton Saunders Ltd of 9 Arkwright Road, Astmoor Industrial Estate, Runcorn,
Cheshire WA7 1NU.
For information in large print, audio CD or braille please contact the Marketing Authorisation Holder.
This leaflet was revised September 2015.
PL 16431/0121
LE1721/02