Potassium Chloride 15% W/V Concentrate For Solution For Infusion
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Potassium Chloride 15% w/v concentrate for solution for infusion
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 1 ml of solution contains 150 mg of potassium chloride (15 % w/v) equivalent to 2 mmol potassium ions.
Each 5 ml of solution contain 750 mg of potassium chloride (15 % w/v) equivalent to 10 mmol potassium ions.
Each 10 ml of solution contain 1500 mg of potassium chloride (15 % w/v) equivalent to 20 mmol potassium ions.
Each 20 ml of solution contain 3000 mg of potassium chloride (15 % w/v) equivalent to 40 mmol potassium ions.
Ionic content: Cl- 2000 mmol/l
K+ 2000 mmol/l
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Concentrate for solution for infusion. A clear colourless solution.
Theoretical osmolarity: 4000 mosm/l pH-value: 4.5 - 7.0
4
CLINICAL PARTICULARS
4.1 Therapeutic indications
Potassium Chloride 150 mg/ml is indicated for the treatment of potassium deficiency in patients for whom dietary measures or oral medication are inadequate.
4.2 Posology and method of administration
INTRAVENOUS ADMINISTRATION ONLY AFTER DILUTION
Posology
Paediatric population dose:
The safety and efficacy of potassium chloride for paediatric patients has not been fully established.
See section 5.1.
Normal dose for adults:
Administer intravenously only after dilution in a suitable solution, up to a maximum concentration of 3 g/l of potassium chloride (or 40 mmol/l of potassium). For therapy of severe hypokalaemia or diabetic ketoacidosis higher concentrations may be necessary; in this case, the infusion should be into a high blood flow vein and continuous ECG monitoring is advisable.
1 g of potassium chloride corresponds to 13.4 mmol or 524 mg of potassium.
Dose is dependent on results of serum electrolyte levels and acid-base-state. The potassium deficit is to be calculated via the following formula:
Potassium deficit (mmol) = kg body weight x 0.2 x 2 x (4.5 mmol/l - serum potassium)
(The extracellular volume calculates from body weight in kg x 0.2.)
Normal daily intake is approximately 0.8 to 2 mmol of potassium per kilogram of body weight.
Infusion rate should not be fast, a rate of 10 mmol/h is normally considered safe.
As a general rule the rate should never be higher than 20 mmol/h.
Usually, the maximum dose for adults should not exceed 150 mmol per day.
Patients with renal impairment
In patients with renal impairment dose should be reduced.
Method of administration
The administration via an infusion pump is recommended, especially for solutions with higher concentrations.
For instructions on dilution of the medicinal product before administration, see section 6.6.
4.3 Contraindications
Potassium Chloride is contraindicated in the following situations:
■ Hyperkalaemia
4.4 Special warnings and precautions for use
Direct injection of potassium chloride concentrates without appropriate dilution may cause instant death.
The administration should be slow (usually 10 mmol/h, not exceeding 20 mmol/h; see section 4.2).
Since adequate urine flow must be ensured, urine flow should be monitored. Care should be taken in patients with uncompensated cardiac insufficiency, in patients under treatment with digitalis and in patients with severe or complete heart block.
Serum electrolyte levels and acid-base status of the patient should be monitored and the dose should be adjusted to the needs of the patient. During treatment, plasma potassium concentration must be measured at regular intervals to avoid the development of hyperkalaemia, especially in patients with renal impairment and other conditions often related with hyperkalaemia. ECG monitoring facilities should be available and patients frequently monitored.
Care should be taken in conditions frequently associated with hyperkalaemia like adrenal insufficiency (Morbus Addison), decreased renal function (renal insufficiency), post-operative oliguria, shock with haemolytic reactions and/or dehydration, metabolic acidosis, patients treated with potassium-sparing diuretics, hyperchloraemia, Gamstorp episodic adynamy, sickle cell anaemia.
Attention should be paid on intravenous administration since extravasation can cause necrotic tissue damages.
Initial potassium replacement therapy should not involve glucose infusions, because glucose may cause a further decrease in the plasma-potassium concentration.
Closely monitor patients with cardiac diseases, acute dehydration, heat cramps, extensive tissue destruction as occurs with severe burns, and elderly patients since renal function may be impaired or other conditions predisposing to hyperkalaemia may be present.
4.5 Interaction with other medicinal products and other forms of interaction
Combinations not recommended (except in cases of severe hypokalaemia):
+ Potassium-sparing diuretics (single or combined) such as: amiloride, spironolactone, triamteren, potassium canrenoate, eplerenone; risk of potentially lethal hyperkalaemia, particularly in patients with renal impairment (addition of hyperkalaemic effects).
+ Angiotensin converting enzyme inhibitors (ACE), angiotensin II receptor antagonists, non-steroidal anti-inflammatory drugs (NSAIDs), cyclosporin, tacrolimus, suxamethonium: potentially lethal hyperkalaemia, particularly in patients with renal insufficiency (addition of hyperkalaemic effects).
+ Blood products, penicillin potassium salts: potential risk of hyperkalaemia due to the amount of potassium present in these products.
Combinations possible with special precautions of use:
+ Quinidine: potassium can increase the anti-arrhythmic effects of quinidine.
+ Thiazides, adrenocorticoids, glucocorticoids, mineralocorticoids:
Effects of the potassium supplement may be decreased.
+ Digoxin: hyperkalaemia can be dangerous in digitalized patients,
+ Exchange resins: the serum levels of potassium are reduced by sodium replacement of the potassium.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data from the use of potassium chloride in pregnant women.
The use of Potassium chloride 150 mg/ml concentrate for solution for infusion may be considered during pregnancy if clinically needed.
Breast-feeding
Potassium chloride is excreted in human milk to such an extent that effects on the breastfed newborn/infants are likely.
A risk to the newborns/infants cannot be excluded.
A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from potassium chloride 150 mg/ml concentrate for solution taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman.
4.7 Effects on ability to drive and use machines
Not relevant.
4.8 Undesirable effects
Excessive intake of potassium may cause hyperkalaemia which may cause neuromuscular and cardiac disorders especially arrhythmias, and even cardiac arrest may occur.
Further undesirable effects:
Metabolism and nutrition disorders:
- acidosis,
- hyperchloraemia.
Vascular disorders:
- venous thrombosis.
General disorders and administration site conditions:
- nausea,
- pain on injection,
- necrosis in case of extravasation,
- phlebitis in case of too high local concentrations.
Overdose causes hyperkalaemia which can produce ECG abnormalities, bradycardia, ventricular fibrillation, other arrhythmias up to cardiac arrest, confusion, tiredness, diarrhoea, dysphagia, paraesthesia of the extremities, respiratory difficulty, skeletal muscles paralysis and death.
When any of these appear, immediately discontinue the treatment and avoid any potassium containing food and potassium-sparing diuretics
In cases of severe hyperkalaemia (over 8 mmol K+/l of plasma) administer i.v. dextrose (10 to 20%) with 10 units of insulin for each 50 g of glucose. Use sodium bicarbonate via i.v. to correct acidosis.
Monitor continuously via ECG. If P wave is absent, administer calcium gluconate 10% (10-20 ml via i.v.).
In order to remove potassium from the body oral sulphonated sodium polystyrene or retention enemas can be used. Haemodialysis or peritoneal dialysis can also be used.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Electrolyte solutions; ATC code: B05XA01.
Mechanism of action
Potassium is the predominant cation within cells and it is involved in numerous cellular functions and metabolic processes. It is essential for carbohydrates metabolism and for the storage of glycogen and also for protein synthesis. It is related to the membrane potential and exerts effects on the muscle, even on heart muscle. Intracellular concentration is about 150 mmol/l, the concentration in plasma is 3.5 -5.5 mmol/l.
Pharmacodynamic effect
Potassium Chloride 150 mg/ml is a concentrate solution of potassium chloride. Potassium chloride helps maintain osmotic pressure and ionic balance. It is essential for intracellular tonicity, transmission of nervous, cardiac and skeletal impulse, and
for the contraction of smooth muscle, renal function, carbohydrates and protein metabolism and multiple enzymatic reactions.
Clinical efficacy and safety
Daily demand is about 1 - 1.5 mmol/kg body weight. Depletion of potassium may occur by increased renal excretion, gastrointestinal loss (vomiting, diarrhoea, fistulae), increased intracellular uptake (treatment of acidosis, glucose-insulin-therapy) or insufficient intake.
Signs of hypokalaemia (below 3.5 mmol/l) are muscle weakness, metabolic alkalosis, impaired renal concentration, intestinal atony with constipation up to paralytic ileus, changes in ECG, and cardiac arrhythmias.
Paediatric population
The safety and efficacy of potassium chloride for paediatric patients has not been fully established.
However, intravenous administration after dilution in a suitable solution up to a maximum dose of 3 mmol of potassium/kg of body weight, or 40 mmol/m2 of body surface is recommended as per available literature. For children weighing 25 kg or over, refer to the adult dosage.
5.2 Pharmacokinetic properties
When administered intravenously, the chloride and potassium ions enter directly into the blood stream and there, as it happens in the mechanism of action, elimination kinetics follows physiologic routes of the body, and it is eliminated in faeces (10 %), urine, sweat, teardrops... It is excreted mainly in urine (90 %).
About 10 - 50 mmol potassium are excreted renally per day, also in potassium depleted patients.
5.3 Preclinical safety data
Potassium chloride is a normal component of human plasma. No toxic foetal or teratogenic effects are known. No carcinogenic effects have been described.
Water for injections.
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
Potassium chloride has been reported to be physically incompatible with the following active substances:
■ amikacin
■ amphotericin B
■ dobutamine
■ fat emulsion
■ mannitol solutions 20% - 25%
■ sodium G penicillin
6.3 Shelf life
Unopened:
5 ml ampoules 2 years 10 ml ampoules 2 years
20 ml ampoules 3 years
Once opened: dilute and use immediately.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
For storage conditions after dilution of the medicinal product, see section 6.3.
6.5 Nature and contents of container
Low density polyethylene (LDPE) ampoules containing 5 ml, 10 ml, and 20 ml.
Package with 20 ampoules containing 5 ml Package with 50 ampoules containing 5 ml Package with 20 ampoules containing 10 ml Package with 50 ampoules containing 10 ml Package with 20 ampoules containing 20 ml
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Potassium Chloride 150 mg/ml is a sterile solution containing potassium chloride for i.v. infusion. It must be diluted before use by not less than 50-times its volume with isotonic solution Sodium Chloride 0.9% w/v intravenous infusion or another suitable solution for infusion.
Compatibility of potassium chloride with any other infusion solution should be established prior to dilution.
In order to avoid a bad homogenization of the diluted solution, the concentrated solution of potassium chloride should not be added to a bottle/bag of infusion in hanging position. Once the concentrated solution has been added to the bottle/bag of infusion, the product must be mixed well before use, so shake the bottle/bag carefully with 3-5 slow movements in order to get a good product homogenization. Then, hang the bottle/bag and start the infusion process.
For single use only. Always use diluted.
Once the ampoule is opened, its spout is perfectly adapted to the Luer syringe and Luer-Lock; therefore, no needle is needed.
To open:
Pull the tab as indicated by the arrow, forward (1) and backward (2), and then twist (3).
Discard the tab (4).
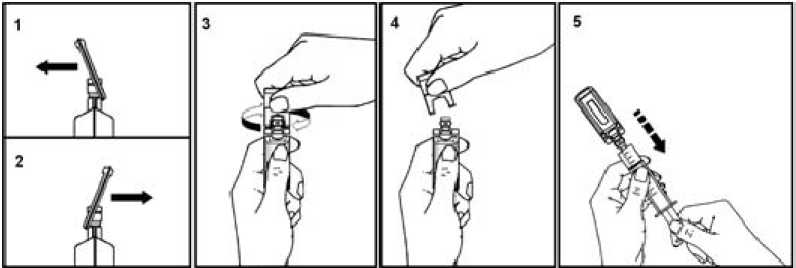
After opening the ampoule, its spout is perfectly adapted to the Luer syringe and Luer-Lock; therefore, no needle is needed.
Connect the syringe to the injectable using a rotating movement. Extract the liquid
(5).
Use in the paediatric population No special requirements.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Fresenius Kabi Ltd Cestrian Court Eastgate Way Manor Park Runcorn Cheshire WA& 1NT
8 MARKETING AUTHORISATION NUMBER(S)
PL 08828/0222
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
DATE OF REVISION OF THE TEXT
10
01/10/2012