Potassium Chloride 15% W/V Concentrate For Solution For Infusion
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Potassium Chloride 15% w/v concentrate for solution for infusion
Potassium chloride.
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Potassium Chloride is and what it is used for
2. What you need to know before you use Potassium Chloride
3. How to use Potassium Chloride
4. Possible side effects
5. How to store Potassium Chloride
6. Contents of the pack and other information
1. What Potassium Chloride is and what it is used for
This product belongs to the group "Additives for intravenous solutions': Electrolytic solutions" and it is dispensed under medical prescription.
Potassium Chloride is indicated for the treatment of potassium deficiency in patients for whom dietary measures or oral medication are inadequate. You must talk to a doctor if you do not feel better or if you feel worse.
2. What you need to know before you use Potassium Chloride
You must not be given Potassium Chloride if you suffer from
excess of potassium in the blood (hyperkalaemia).
Warnings and Precautions
Potassium Chloride will be given by or under the direct
supervision of your physician, who will take care for the following:
- Direct injection of potassium chloride concentrates without appropriate dilution may cause instant death.
- The administration should be slow (usually 10 mmol/hr, not exceeding 20 mmol/hr) and under control of cardiac activity.
- Since adequate urine flow must be ensured, your urine flow should be monitored.
- Your serum electrolyte levels (serum ionogram) and acid-base status should be monitored and the dose should be adjusted to your individual needs.
- Closely monitor patients with diseases of the heart, acute fluid deficiency (acute dehydration), heat cramps, extensive tissue destruction as occurs with severe burns, and elderly patients since renal function may be impaired or other conditions predisposing to excess of potassium in the blood (hyperkalaemia) may be present.
- Initial potassium replacement therapy should not involve glucose infusions, because glucose may cause a further decrease in the plasma-potassium concentration.
- If signs of renal failure occur, intravenous administration of potassium-containing solutions should be stopped.
Your doctor may need to take special precautions and will decide whether you can receive Potassium Chloride if you suffer from:
- uncompensated heart failure (decompensated cardiac insufficiency), if you are under treatment with digitalis (medicines used for the treatment of diseases of the heart), and if you have severe or complete heart block.
- conditions which are frequently associated with an excess of potassium in the blood (hyperkalaemia): Gamstorp episodic adynamy (a form of periodic paralysis), sickle cell anaemia, impaired function of the adrenal glands (adrenal insufficiency), decreased renal function (renal insufficiency), low urinary output after surgery (post-operative oliguria), shock with disintegration of red blood cells and/or deficiency of body fluid (shock with haemolytic reactions and/ or dehydration), metabolic acidosis (form of acidic blood), treatment with potassium-sparing diuretics (medicines used to increase urine excretion that retain potassium in the blood), excess of chloride in the blood (hyperchloraemia).
Your doctor should pay attention on intravenous administration since leakage of infusion fluid out of the vessel (extravasation) can cause tissue death (necrotic tissue damages).
Children The safety and efficacy of potassium chloride for paediatric patients has not been fully established.
Other medicines and Potassium chloride:
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Combinations not recommended (except in cases of severe potassium deficiency):
+ Potassium-sparing diuretics (medicines used to increase urine excretion that retain potassium in the blood), single or combined such as: amiloride, spironolactone, triamteren, potassium canrenoate, eplerenone; risk of potentially fatal excess of potassium in the blood (hyperkalaemia), particularly in patients with renal impairment (addition of potassium increasing effects). + Angiotensin converting enzyme inhibitors (ACE), angiotensin II receptor antagonists, non-steroidal anti-inflammatory drugs (NSAIDs), cyclosporin, tacrolimus, suxamethonium: potentially fatal excess of potassium in the blood (hyperkalaemia),

particularly in patients with renal impairment (addition of potassium increasing effects).
+ Blood products, penicillin potassium salts: potential risk of potassium excess in the blood (hyperkalaemia) due to the amount of potassium present in these products.
Combinations possible with special precautions of use:
+ Quinidine: potassium can increase the anti-arrhythmic effects of quinidine.
+ Thiazides, adrenocorticoids, glucocorticoids, mineralocorticoids: Effects of the potassium supplement may be decreased.
+ Digoxin: excess of potassium in the blood (hyperkalaemia) can be dangerous if you take digitalis medicines for the treatment of diseases of the heart,
+ Exchange resins: the serum levels of potassium are reduced by replacing potassium with sodium.
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
However physical incompatibility of Potassium Chloride 150mg/ ml concentrate for solution for infusion has been reported with the following medicinal substances: amikacin, amphotericin B, dobutamine, fat emulsions, mannitol solutions 20% - 25% and sodium G penicillin.
Pregnancy, breast-feeding and fertility
Ask your doctor or pharmacist for advice before taking any medicine.
There are no or limited amount of data from the use of potassium chloride in pregnant women.
The use of Potassium chloride 15% w/v concentrate for solution for infusion may be considered during pregnancy if clinically needed.
Potassium chloride is excreted in human milk to such an extent that effects on the breastfed newborn/infants are likely.
A risk to the newborns/infants cannot be excluded.
Your doctor must decide whether to discontinue breast-feeding or to discontinue/abstain from Potassium Chloride 15% w/v concentrate for solution for infusion taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask our doctor or pharmacist for advice before taking this medicine.
Driving and using machines
There is no evidence that this product could affect the ability to drive vehicles or manipulate complex machinery.
3. How to use Potassium Chloride
Potassium Chloride will be given by, or under direct supervision of your physician, who will closely control the amount of Potassium Chloride given to you.
Your doctor will decide on the correct dose for you to receive.
Normal dose for adults and adolescents:
Administer intravenously only after dilution in a suitable solution, up to a maximum concentration of 3 g/l of potassium chloride (or 40 mmol/l of potassium). For therapy of severe hypokalaemia or diabetic ketoacidosis higher concentrations may be necessary. In this case, the infusion should be into a high blood flow vein and continuous ECG monitoring is advisable.
1 g of potassium chloride corresponds to 13.4 mmol or 524 mg of potassium.
Dose is dependent on results of serum ionogram and acid-base-state. The potassium deficit is to be calculated via the following formula:
Potassium deficit (mmol) = kg body weight x 0.2 x 2 x (4.5 mmol/l -serum potassium)
(The extracellular volume calculates from body weight in kg x 0.2.) Normal daily intake is approximately 0.8 to 2 mmol of potassium per kilogram of body weight.
Usually, the maximum dose for adults should not exceed 150 mmol per day.
Paediatric population dose:
Intravenous administration after dilution in a suitable solution up to a maximum dose of 3 mmol of potassium/kg of body weight, or 40 mmol/m2 of body surface is recommended. For children weighing 25 kg or over, refer to the adult dosage.
Maximum dose for children is 3 mmol/kg of body weight and day. In patients with renal impairment dose should be reduced.
Route of administration:
You will receive this medicines diluted by infusion into a vein (intravenous drip). The infusion rate will be slow, the amount of potassium chloride will depend on your specific requirement.
A rate of 10 mmol/h is normally considered safe. As a general rule the rate should never be higher than 20 mmol/h.
The administration via an infusion pump is recommended, especially for solutions with higher concentrations.
Your doctor will tell you how long the treatment with Potassium Chloride should last.
If you think that the action of Potassium Chloride is too strong or weak tell your doctor or pharmacist.
If you receive more Potassium Chloride than you should:
Overdose as a result of potassium excess in the blood can produce ECG abnormalities, lowered heart rate (bradycardia), irregular heart rhythm with very rapid, uncoordinated, fluttering contractions of the lower chambers of the heart (ventricular fibrillation), other rhythm disorders of the heart (arrhythmias) up to cardiac arrest, confusion, tiredness, diarrhoea, swallowing disorders, abnormal skin sensations felt in the arms or legs (paraesthesia of the extremities), breathing difficulty, skeletal muscles paralysis, and death.
V003
If any of these effects appear, immediately stop the treatment and avoid any potassium containing food and potassium-sparing diuretics (medicines used to increase urine excretion that retain potassium in the blood).
In case of overdose or accidental use, contact an emergency centre immediately indicating the medicine and guantity used.
If you experience any of these symptoms or think you may have received too much Potassium Chloride, tell your doctor or healthcare personnel immediately.
If you have any further guestions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Potassium Chloride can cause side effects, although not everybody gets them.
Excessive intake of potassium may cause potassium excess in the blood (hyperkalaemia) which may cause neuromuscular and heart disorders, especially rhythm disorders, and even cardiac arrest can occur.
Further undesirable effects:
Metabolism and nutrition disorders:
- acidic blood (acidosis),
- excess of chloride in the blood (hyperchloraemia).
Vascular disorders:
- blood clot inside a blood vessel (venous thrombosis).
General disorders and administration site conditions:
- nausea
- pain at the injection site,
- cellular death incase of leakage of infusion fluid out of the vessel (extravasation),
- venous inflammation in case of too high local concentrations.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly:
For UK: via the Yellow Card Scheme at: "http://www.mhra.gov.uk/yellowcard"
For IE: Reports may be made by following the links to the online reporting option accessible from the IMB homepage, or by completing the downloadable report form also accessible from the IMB website, which may be completed manually and submitted to the IMB via freepost, to the following address:
FREEPOST
IMB Pharmacovigilance Earlsfort Terrace IRL-Dublin 2
Tel: +3531 6764971, Fax: +3531 6762517 Website: http://www.imb.ie e-mail: imbpharmacovigilancedimb.ie.
5. How to store Potassium Chloride
Keep out of the sight and reach of children.
Do not use Potassium Chloride 15% w/v concentrate for solution for infusion after the expiry date which is stated on the ampoule and outer carton after EXP. The expiry date refers to the last day of that month.
This medicinal product does not reguireany special storage conditions.
Do not use Potassium Chloride 15% w/v concentrate for solution for infusion if the solution is cloudy, contains any visible particle, or shows discolouration.
Do not throw away any medicine via wastewater or household waste. Ask you pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Potassium chloride contains
The active substance is potassium chloride.
Each 1 ml of solution contains 150 mg of potassium chloride (15 % w/v) eguivalent to 2 mmol potassium ions.
Each 5 ml of solution contain 750 mg of potassium chloride (15 % w/v) eguivalent to 10 mmol potassium ions.
Each 10 ml of solution contain 1500 mg of potassium chloride (15 % w/v) eguivalent to 20 mmol potassium ions.
Each 20 ml of solution contain 3000 mg of potassium chloride (15 % w/v) eguivalent to 40 mmol potassium ions.
Ionic content: K+ 2000 mmol/l Cl- 2000 mmol/l
Theoretical osmolarity: 4000 mosm/l
What Potassium Chloride 15% w/v concentrate for solution for infusion looks like and contents of the pack
Potassium Chloride 15% w/v concentrate for solution for infusion is a clear, colourless solution.
Potassium Chloride 15% w/v concentrate for solution for infusion is presented in the following formats:
Package with 20 ampoules containing 5 ml Package with 50 ampoules containing 5 ml Package with 20 ampoules containing 10 ml Package with 50 ampoules containing 10 ml Package with 20 ampoules containing 20 ml Not all pack sizes may be marketed.
Instructions for the correct administration:
Potassium Chloride 15% w/v is a sterile solution containing potassium chloride for i.v. infusion. It must be diluted by not less than 50-times its volume with isotonic Sodium Chloride 0.9% w/v solution for infusion or another suitable solution for infusion.

FHandling instructions
This leaflet was last approved in 01/2013
Latvia
Lithuania
Poland
Portugal
Romania
Spain
United
Kingdom

Compatibility of Potassium Chloride with any other infusion solution should be established prior to dilution.
In order to avoid a bad homogenization of the diluted solution, the concentrated solution of Potassium Chloride should not be added to a bottle/bag of infusion in hanging position. Once the concentrated solution has been added to the bottle/bag of infusion, the product must be mixed well before use, so shake the bottle/bag carefully with 3-5 slow movements in order to get a good product homogenization. Then, hang the bottle/bag and start the infusion process.
For single use only. Always use diluted!
Potassium chloride Kabi 150 mg/ml koncentrats infuziju skiduma pagatavosanai
Potassium chloride Kabi 150 mg/ml koncentratas infuziniam tirpalui
Kalium chloratum 15% Kabi Cloreto de Potassio Kabi Clorura de potasiu Kabi 150 mg/ml, concentrat pentru solutie perfuzabila
Cloruro de potasio Meinsol 2 mEg/ml, concentrado para solucion para perfusion
Potassium Chloride 15% w/v concentrate for solution for infusion
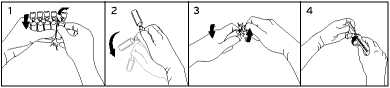
To break off a single ampoule, twist one ampoule against the remaining ampoules of the pack without touching the head and neck of the ampoules (1). Shake the ampoule with one single movement as shown below in order to remove the liguid kept in the cap (2). To open the ampoule, twist the ampoule body and the ampoule head in opposite directions until the neck breaks off (3). Connect the ampoule to the luer-syringe or luer-lock syringe as shown in figure (4).
Marketing Authorisation Holder and Manufacturer:
Marketing Authorisation Holder
Fresenius Kabi Ltd
Cestrian Court, Eastgate Way, Manor Park, Runcorn, Cheshire, WA71NT.UK
Manufacturer:
FRESENIUS KABI ESPANA.S.A.
C/Marina 16-18 08005 Barcelona, Spain
This medicinal product is authorised in the Member States of the EEA under the following names:
Belgium Kaliumchloride Fresenius Kabi 150 mg/ml
concentraat voor oplossing voor infusie Estonia Potassium chloride Kabi 150 mg/ml
Germany Kaliumchlorid Kabi 150 mg/ml Konzentrat zur Herstellung einer Infusionslosung
Greece Potassium Chloride/Fresenius, nuKvo draXupta
yia tr|v IlapasKeurj diaAvpiatoc; ppoc;
FRESENIUS
KABI
£YXl'srF 2M.
Ireland Potassium Chloride 15% w/v
concentrate for solution for infusion