Pregabalin Focus 20 Mg/Ml Oral Solution
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again. If you have further questions, ask your doctor or your pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Pregabalin oral solution is and what it is used for
2. What you need to know before you take Pregabalin oral solution
3. How to take Pregabalin oral solution
4. Possible side effects
5. How to store Pregabalin oral solution
6. Contents of the pack and other information
1. What Pregabalin oral solution is and what it is used for
Pregabalin oral solution belongs to a group of medicines used to treat epilepsy and Generalised Anxiety Disorder (GAD) in adults.
Epilepsy: Pregabalin oral solution is used to treat a certain form of epilepsy (partial seizures with or without secondary generalisation- epileptic fits starting on one specific part of the brain) in adults. Your doctor will prescribe Pregabalin oral solution for you to help treat your epilepsy when your current treatment is not controlling your condition. You should take Pregabalin oral solution in addition lo your current treatment. Pregabalin oral solution s not intended to be used alone, but should always be used in combination with other antiepileptic treatment.
Generalised Anxiety Disorder: Pregabalin oral solution is used to treat Generalised Anxiety Disorder (GAD). The symptoms of GAD are orolonged excessive anxiety and worry that are difficult to control. GAD can also cause restlessness or feeling keyed up or on edge, being easily fatigued (tired), having difficulty concentrating or mind going blank, feeling irritable, having muscle tension or sleep disturbance. This is different to the stresses and strains of everyday life.
2. What you need to know before you take Pregabalin oral solution
Do not take Pregabalin oral solution
If you are allergic to pregabalin or any of the other ngredients of this medicine (listed in section 6). Warnings and Precautions Talk to your doctor or pharmacist before taking Pregabalin oral solution.
• Some patients taking Pregabalin oral solution have reported symptoms suggesting an allergic reaction. These symptoms include swelling of the face, lips, tongue, and throat, as well as diffuse skin rash. Should you experience any of these reactions, you should contact your physician immediately.
• Pregabalin oral solution has been associated with dizziness and somnolence, which could increase the occurrence of accidental injury (fall) in elderly patients. Therefore, you should be careful until you are used to any effect the medicine might have.
• Pregabalin oral solution may cause blurring or loss of vision, or other changes in eyesight, many of which are temporary. You should immediately tell your doctor if you experience any changes in your vision.
• Some patients with diabetes who gain weight while taking pregabalin may need an alteration in their diabetic medicines.
• Certain side effects may be more common, such as sleepiness, because patients with spinal cord injury may be taking other medicines to treat, for example, pain or spasticity, that have similar side effects to Pregabalin and the severity of these effects may be increased when taken together.
• There have been reports of heart failure in some patients when taking Pregabalin oral solution; these patients were mostly elderly with cardiovascular conditions. Before taking this medicine you should tell your doctor if you have a history of heart disease.
• There have been reports of kidney failure in some patients when taking Pregabalin oral solution. If while taking Pregabalin oral solution you notice decreased urination, you should tell your doctor as stopping the medicine may improve this.
• A small number of people being treated with anti-epileptics such as Pregabalin oral solution have had thoughts of harming or killing themselves. If at any time you have these thoughts, immediately contact your doctor.
• When Pregabalin oral solution is taken with other medicines that may cause constipation (such as some types of pain medicines) it is possible that gastrointestinal problems may occur (e.g. constipation, blocked or paralysed bowel). Tell your doctor if you experience constipation, especially if you are prone to this problem.
• Before taking this medicine you should tell your doctor if you have a history of alcoholism or any drug abuse or dependence. Do not take more medicine than prescribed.
• There have been reports of convulsions when taking Pregabalin oral solution or shortly after stopping Pregabalin oral solution. If you experience a convulsion, contact your doctor immediately.
• There have been reports of reduction in brain function (encephalopathy) in some patients taking Pregabalin oral solution when they have other conditions. Tell your doctor if you have a history of any serious medical conditions, including liver or kidney disease.
Children and adolescents
The safety and efficacy in children and
adolescents (under 18 years of age) has not been established and therefore, pregabalin should not be used in this age group.
Other medicines and Pregabalin oral solution
Please tell your doctor or pharmacist if you are taking, have recently taken any other medicines, including medicines obtained without a prescription.
Pregabalin oral solution and certain other medicines may influence each other (interaction). When taken with certain other medicines, Pregabalin oral solution may potentiate the side effects seen with these medicines, including respiratory failure and coma. The degree of dizziness, sleepiness and decreased concentration may be increased if Pregabalin oral solution is taken together with medicinal products containing:
Oxycodone - (used as a pain-killer)
Lorazepam - (used for treating anxiety)
Alcohol
Pregabalin oral solution may be taken with oral contraceptives.
Pregabalin oral solution with food, drink and alcohol
Pregabalin oral solution may be taken with or without food.
It is advised not to drink alcohol while taking Pregabalin oral solution
Pregnancy and breast-feeding
Pregabalin oral solution should not be taken during pregnancy or when breast-feeding, unless you are told otherwise by your doctor. Effective contraception must be used by women of childbearing potential. If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Driving and using machines Pregabalin oral solution may produce dizziness, sleepiness and decreased concentration. You should not drive, operate complex machinery or engage in other potentially hazardous activities until you know whether this medicine affects your ability to perform these activities.
Pregabalin oral solution contains Pregabalin oral solution oral solution contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions (possibly delayed).
3. How to take Pregabalin oral solution
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacisl if you are not sure.
Your doctor will determine what dose is appropriate for you.
Epilepsy or Generalised Anxiety Disorder:
• Take the solution as instructed by your doctor.
• The dose, which has been adjusted for you and your condition, will generally be between 150 mg (7.5 ml) and 600 mg (30 ml) each day.
• Your doctor will tell you to take Pregabalin oral solution either twice or three times a day. For twice a day take Pregabalin oral solution once in the morning and once in the evening, at about the same time each day. For three times a day take Pregabalin oral solution once in the morning, once in the afternoon and once in the evening, at about the same time each day.
If you have the impression that the effect of Pregabalin oral solution is too strong or too weak, talk to your doctor or pharmacist.
If you are an elderly patient (over 65 years of age), you should take Pregabalin oral solution normally except if you have problems with your kidneys.
Your doctor may prescribe a different dosing schedule and/or dose if you have problems with your kidneys.
Continue taking Pregabalin oral solution until your doctor tells you to stop.
Administration:
Instructions for use
Pregabalin oral solution is for oral use only.
1. Open the bottle: Press downward on the cap and turn it counter-clockwise (Figure 1).
2. First time use only: A Press-In Bottle Adapter (PIBA) is provided with the oral syringe. This is the device that gets inserted into the neck of the bottle to make it easier to withdraw the solution using the oral syringe. With the bottle on a flat surface, insert the PIBA into the bottle neck while keeping the PIBA's flat surface facing up and pressing on it (Figure 2).
3. Push the syringe plunger to the bottom of the barrel of the syringe (toward its tip) to remove excess air. Attach the syringe to the PIBA with a slight twisting motion (Figure 3).
4. Invert the bottle (with the syringe attached) and fill the syringe with the liquid by pulling the syringe plunger down to just beyond the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (Figure 4). Remove air bubbles from the syringe by pushing the plunger up to the appropriate graduation mark.
5. Return the bottle to an upright position with the syringe still in the PIBA/bottle (Figure 5).
6. Remove the syringe from the bottle/PIBA (Figure 6).
7. Empty the contents of the syringe directly into mouth by pushing the syringe plunger to the bottom of the syringe barrel (Figure 7).
Note: Steps 4-7 may need to be repeated up to three times to obtain the total dose (Table 1).
[For example, a 150 mg (7.5 ml) dose will require two withdrawals from the bottle to achieve the entire dose. Using the oral syringe, first withdraw 5 ml and empty contents of syringe directly into the mouth, then refill the oral syringe with 2.5 ml and empty the remaining contents into the mouth.]
8. Rinse the syringe by drawing water into the syringe and pushing the syringe plunger to the bottom of the syringe barrel, at least three times (Figure 8).
|
r |
\ |
|
FOCUS | |
|
Version No: |
103536/LF/046/01 |
|
Product Name: |
Pregabalin Focus |
|
20 mg/ml oral solution | |
|
Pack Size: |
2 x 250 ml |
|
Component: |
Leaflet |
|
SKU: |
103536 |
|
Market: |
UK |
|
Production Site: |
Lamda Laboratories SA |
|
Revision No.: |
7 |
|
Revision Date: |
02/09/2016 |
|
Revised by: |
ADD |
|
CRF: |
AMCO.CRF.166.2015 |
|
PR 6196 | |
|
V_ |
y |
|
Dimension: |
175 x 500 mm |
|
Commodity No.: |
N/A |
|
Pharma Code: |
N/A |
|
Core Spec Ref: |
N/A |
|
DCMF: |
N/A |
|
Print Colours: |
Black |
|
Non-Print Colours: |
Cutter |
|
Tech App. Date: |
06/05/2016 |
|
Min. Font Size: |
8 pt |
|
V |
J |
/■ \ REGULATORY AUTHORITY APPROVAL CONFIRMATION
Confirmation that this artwork has been approved by the appropriate market authority (if applicable, e.g. MHRA, HPRA, etc) and that Mercury Pharma have license approval to distribute this component for sale in the relevant market.
Signature
Name
Date
|
V_ | ||
|
r V |
PAGE 1 OF 2 |
) |
9. Replace the cap on the bottle (leaving the PIBA in place in the bottle neck) (Figure 9).
Table 1. Oral syringe withdrawals to Deliver Prescribed Dose of Pregabalin 20mg/ml oral solution
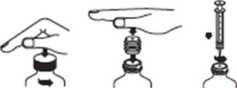
Figure 1
Figure 3
Figure 2
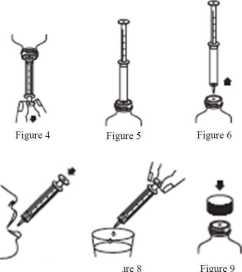
Figure 7 Figi
|
Pregabalin Oral solution Dose (mg) |
Total Solution Volume (ml) |
First Syringe Withdrawal (ml) |
Second Syringe Withdrawal (ml) |
Third Syringe Withdrawal (ml) |
|
25 |
1.25 |
1.25 |
Not required |
Not required |
|
50 |
2.5 |
2.5 |
Not required |
Not required |
|
75 |
3.75 |
3.75 |
Not required |
Not required |
|
100 |
5 |
5 |
Not required |
Not required |
|
150 |
7.5 |
5 |
2.5 |
Not required |
|
200 |
10 |
5 |
5 |
Not required |
|
225 |
11.25 |
5 |
5 |
1.25 |
|
300 |
15 |
5 |
5 |
5 |
If you take more Pregabalin oral solution than you should
Call your doctor or go to the nearest hospital emergency unit immediately. Take your box or Dottle of Pregabalin oral solution with you. You may Feel sleepy, confused, agitated, or restless as a "esult of taking more Pregabalin oral solution than you should.
If you forget to take Pregabalin oral solution
t is important to take your Pregabalin oral solution 'egularly at the same time each day. If you forget to take a dose, take it as soon as you remember unless it is time for your next dose. In that case, just carry on with the next dose as normal. Do not take a double dose to make up for a forgotten dose.
If you stop taking Pregabalin oral solution Do not stop taking Pregabalin oral solution unless your doctor tells you to. If your treatment is stopped it should be done gradually over a minimum of 1 week.
After stopping long and short-term Pregabalin oral solution treatment, you need to know that you may experience certain side effects. These include, trouble sleeping, headache, nausea, feeling anxious, diarrhoea, flu-like symptoms, convulsions, nervousness, depression, pain, sweating, and dizziness. These symptoms may occur more commonly or severely if you have been taking 3regabalin oral solution for a longer period of time, f you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
_ike all medicines, this medicine can cause side effects, although not everybody gets them.
Very common: may affect more than 1 in 10 people
Dizziness, drowsiness, headache
Common: may affect up to 1 in 10 people
Increased appetite
Feeling of elation, confusion, disorientation, decrease in sexual interest, irritability Disturbance in attention, clumsiness, memory impairment, loss of memory, tremor, difficulty with speaking, tingling feeling, numbness, sedation, lethargy, insomnia, fatigue, feeling abnormal
Blurred vision, double vision Vertigo, problems with balance, fall Dry mouth, constipation, vomiting, flatulence, diarrhoea, nausea, swollen abdomen Difficulties with erection Swelling of the body including extremities Feeling drunk, abnormal style of walking Weight gain
Muscle cramp, joint pain, back pain, pain in limb
Sore throat
Uncommon: may affect up to 1 in 100 people
Loss of appetite, weight loss, low blood sugar, high blood sugar
Change in perception of self, restlessness, depression, agitation, mood swings, difficulty finding words, hallucinations, abnormal dreams, panic attacks, apathy, aggression, elevated mood, mental impairment, difficulty with thinking, increase in sexual interest, problems with sexual functioning including inability to achieve a sexual climax, delayed ejaculation Changes in eyesight, unusual eye movement, changes in vision including tunnel vision, flashes of light, jerky movements, reduced reflexes, increased activity, dizziness on standing, sensitive skin, loss of taste, burning sensation, tremor on movement, decreased consciousness, loss of consciousness, fainting, increased sensitivity to noise, feeling unwell Dry eyes, eye swelling, eye pain, weak eyes, watery eyes, eye irritation Heart rhythm disturbances, increased heart rate, low blood pressure, high blood pressure,
changes in heart beat, heart failure Flushing, hot flushes
Difficulty breathing, dry nose, nasal congestion Increased saliva production, heartburn, numb around mouth Sweating, rash ,chills, fever Muscle twitching, joint swelling, muscle stiffness, pain including muscle pain, neck pain Breast pain
Difficulty with or painful urination, incontinence Weakness, thirst, chest tightness Changes in blood and liver test results (blood creatinine phosphokinase increased, alanine amino transferase increased, aspartate aminotransferase increased, platelet count decreased, neutropenia, increase in blood creatinine, decrease in blood potassium) Hypersensitivity, swollen face, itchiness, hives, runny nose, nose bleed, cough, snoring Painful menstrual periods Coldness of hands and feet.
Rare: may affect up to 1 in 1,000 people:
Abnormal sense of smell, swinging vision, altered perception of depth, visual brightness, vision loss Dilated pupils, cross eyes Cold sweat, tightness of the throat, swollen tongue
Inflammation of the pancreas Difficulty in swallowing Slow or reduced movement of the body Difficulty with writing properly.
Increased fluid in the abdomen Fluid in the lungs Convulsions
Changes in the recording of electrical changes (ECG) in the heart which correspond to heart rhythm disturbances Muscle damage
Breast discharge, abnormal breast growth, breast growth in males Interrupted menstrual periods Kidney failure, reduced urine volume, urinary retention
Decrease in white blood cell count Inappropriate behavior Allergic reactions (which may include difficulty breathing, inflammation of the eyes (keratitis) and a serious skin reaction characterized by rash, blisters, peeling skin and pain)
If you experience swollen face or tongue or if your skin turns red and starts to blister or peel you should seek immediate medical advice.
Certain side effects may be more common, such as sleepiness, because patients with spinal cord injury may be taking other medicines to treat, for example, pain or spasticity, that have similar side effects to pregabalin oral solution and the severity of these effects may be increased when taken together.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store pregabalin oral solution
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton or bottle. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
After first opening do not store above 25°C and use within 3 months.
Do not throw away medicine via wastewater. Ask your pharmacist how to throw away medicine you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information What Pregabalin oral solution contains
The active substance is pregabalin. Each ml contains 20 mg of pregabalin.
The other ingredients are: methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), sodium dihydrogen phosphate dihydrate, disodium phosphate, anhydrous (E339), sucralose (E955), strawberry flavor, sodium hydroxide 1N (for pH adjustment), purified water.
What Pregabalin oral solution looks like and contents of the pack
Pregabalin oral solution 20 mg/ml oral solution is a clear colourless solution with characteristic strawberry odour in white HDPE bottles containing 250 ml of oral solution, in a cardboard carton. The carton contains two HDPE bottles of 250 ml nominal capacity with HDPE - lined tamper evident and child-resistant screw caps. The carton also contains a 5 ml, CE marked oral syringe with intermediate graduations of 0.25 ml and two press-in bottle adaptors (PIBA).
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Focus Pharmaceuticals Ltd.,
Capital House, 85 King William Street London, EC4N 7BL, United Kingdom
Manufacturer:
Anfarm Hellas SA Sximatari Viotias,32009, Greece Tel:+30.22620.58678 / +30.22620.58391 fax: +30.22620.58392
This leaflet was last revised in September 2016.
103536/LF/046/01
|
Version No: |
103536/LF/046/01 |
|
Product Name: |
Pregabalin Focus 20 mg/ml oral solution |
|
Pack Size: |
2 x 250 ml |
|
Component: |
Leaflet |
|
SKU: |
103536 |
|
Market: |
UK |
|
Production Site: |
Lamda Laboratories S.A |
|
Revision No.: |
7 |
|
Revision Date: |
02/09/2016 |
|
Revised by: |
ADD |
|
CRF: |
AMCO.CRF.166.2015 PR 6196 |
|
Dimension: |
175 x 500 mm |
|
Commodity No.: |
N/A |
|
Pharma Code: |
N/A |
|
Core Spec Ref: |
N/A |
|
DCMF: |
N/A |
|
Print Colours: |
Black |
|
Non-Print Colours: |
Cutter |
|
Tech App. Date: |
06/05/2016 |
|
Min. Font Size: |
8 pt |
REGULATORY AUTHORITY APPROVAL CONFIRMATION
Confirmation that this artwork has been approved by the appropriate market authority (if applicable, e.g. MHRA, HPRA, etc) and that Mercury Pharma have license approval to distribute this component for sale in the relevant market.
Accept Artwork / Reject Artwork
(Please strike off whichever NOT applicable)
Signature
Name
Date