Pregnyl 1500 Iu Powder For Solution For Injection
Bar prints 100mm @ 100%

Black Cyan

Magenta

Warm Red

Technical Info Profile
11022/150915-2
^ MSD
Pregnyl® 1500 I.U.
powder for solution for injection
(Human Chorionic Gonadotrophin)
INFORMATION FOR THE DOCTOR
1. NAME OF THE MEDICINAL PRODUCT
Pregnyl® 1500 I.U. powder for solution for injection.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Pregnyl consists of a freeze-dried powder for injection. The active ingredient [human chorionic gonadotrophin (hCG)] which is obtained from the urine of pregnant women, has luteinizing hormone (LH) activity.
An ampoule contains 1500 I.U. hCG. For a full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Powder for solution for injection. The powder is a white, dry powder or cake.
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
In the female
Sterility due to the absence of follicle-ripening or ovulation.
In combination with FSH or HMG, promotion of controlled superovulation in medically assisted reproduction programmes.
In the male
Hypogonadotrophic hypogonadism. Delayed puberty associated with insufficient gonadotrophic pituitary function.
Sterility in selected cases of deficient spermatogenesis.
4.2 Posology and method of administration
Posology In the female
Sterility due to the absence of follicle-ripening or ovulation.
5.000- 10,000 IU hCG to induce ovulation, following treatment with an FSH (Follicle Stimulating Hormone)
or HMG (Human Menopausal Gonadotrophins) preparation.
In combination with FSH or HMG, promotion of controlled superovulation in medically assisted reproduction programmes.
5.000- 10,000 IU hCG 30 - 40 hours after the last FSH or HMG injection. Pregnyl should not be administered
if the following criteria have not been met: at least 3 follicles greater than 17 mm in diameter are present with 17p estradiol levels of at least 3500 pmol/L (920 picogram/ml). Oocyte collection is carried out 32 - 36 hours after the hCG injection. Os luteal phase support, two to three injections of 1,000 to 3,000 IU hCG each may be given within nine days of ovulation or embryo transfer, for example on day 3, 6 and 9 after ovulation induction or embryo transfer. In the male
Hypogonadotrophic hypogonadism. 500-1,000 IU hCG 2-3 times weekly. Delayed puberty associated with insufficient gonadotrophic pituitary function.
1,500 IU hCG twice weekly for at least 6 months.
Sterility in selected cases of deficient spermatogenesis.
Usually, 3,000 IU hCG per week in combination with an FSH or HMG preparation.
This treatment should be continued for at least three months before any improvement in spermatogenesis can be expected. During this treatment testosterone replacement therapy should be suspended. Once achieved, the improvement may sometimes be maintained by hCG alone.
Method of Administration
After addition of the solvent to the freeze-dried substance, the solution should be given immediately by intramuscular or subcutaneous injection. Any unused solution should be discarded. Subcutaneous injection may be carried out by patient or partner, provided that proper instruction is given by the physician. Self administration of Pregnyl should only be performed by patients who are well-motivated, adequately trained and with access to expert advice.
4.3 Contraindications
■ Hypersensitivity to human gonadotrophins or any of the excipients listed in section 6.1.
■ Presence of uncontrolled non-gonadal endocrinopathies (e.g. thyroid, adrenal or pituitary disorders).
■ Breast, uterine, ovarian tumours.
■ Vaginal bleeding of unknown cause.
■ Known or suspected androgen-dependent tumours such as testicular tumours, carcinoma of the prostate or mammary carcinoma in males.
■ Malformations of the sexual organs incompatible with pregnancy.
■ Fibroid tumours of the uterus incompatible with pregnancy.
4.4 Special warnings and precautions for use
In the female
■ Since infertile women undergoing assisted reproduction, and particularly IVF, often have tubal abnormalities the incidence
of ectopic pregnancies might be increased. Early ultrasound confirmation that a pregnancy is intrauterine is therefore important.
■ Prior to treating patients for inadequate endogenous stimulation of the gonads, an examination should be performed to exclude anatomical abnormalities of the genital organs or nongonadal endocrinopathies (e.g. thyroid or adrenal disorders, diabetes). Primary ovarian failure should be excluded by the determination of gonadotrophin levels.
■ In the pregnancies occurring after induction of ovulation with gonadotrophic preparations, there is an increased risk of abortion and multiplets. Multiple pregnancy, especially high order, carries an increased risk in adverse maternal and perinatal outcomes. The parents should be advised of the potential risks of multiple births before starting treatment.
■ The incidence of congenital malformations after Assisted Reproductive Technologies (ART) may be higher than after spontaneous conceptions. This is thought to be due to differences in parental characteristics (e.g. maternal age, sperm characteristics) and an increased incidence of multiple gestations.
■ Women with generally recognised risk factors for thrombosis,
such as a personal or family history, severe obesity (Body Mass Index > 30 kg/m2) or thrombophilia, may have an increased risk of venous or arterial thromboembolic events, during or following treatment with gonadotrophins. In these women the benefits of IVF treatment need to be weighed against the risks.
It should be noted, however, that pregnancy itself also carries an increased risk of thrombosis.
■ There have been reports of ovarian and other reproductive system neoplasms, both benign and malignant, in women
who have undergone multiple drug regimens for infertility treatment. It is not yet established whether or not treatment with gonadotrophins increases the baseline risk of these tumours in infertile women.
Unwanted Hyperstimulation During treatment of female patients, determinations of oestrogen levels and assessment of ovarian size and if possible, ultrasonography should be performed prior to treatment and at regular intervals during treatment. High dosages may cause oestrogen levels to rise excessively rapidly, e.g. more than doubling on 2 or 3 consecutive days, and possibly reaching excessively high pre-ovulatory values. The diagnosis of unwanted ovarian hyperstimulation may be confirmed by ultrasound examination.
If unwanted hyperstimulation occurs (i.e. not as part of a treatment preparing for IVF/ET or GIFT or other assisted reproduction techniques), the administration of FSH or HMG should be discontinued immediately. HCG must not be given, because the administration of an hLH - active gonadotrophin at this stage may induce, in addition to multiple ovulations, the ovarian hyperstimulation syndrome. This warning is particularly important with respect to patients with polycystic ovarian disease.
Clinical symptoms of mild ovarian hyperstimulation syndrome include gastro-intestinal problems (pain, nausea, diarrhoea, abdominal discomfort and bloating), painful breasts, and mild to moderate enlargement of ovaries and ovarian cysts. Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with ovarian hyperstimulation syndrome.
The severe form of ovarian hyperstimulation syndrome may be life-threatening and is characterised by large ovarian cysts (prone to rupture), acute abdominal pain, ascites, weight gain, very often hydrothrax and occasionally thrombo-embolic phenomena.
Pregnyl should not be used for body weight reduction. HCG has no effect on fat metabolism, fat distribution or appetite.


Package Leaflet: Information for user
Pregnyl® 1500 I.U.
powder for solution for injection
(Human Chorionic Gonadotrophin)
Read all of this leaflet carefully
before you start taking this medicine
because it contains important
information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you. Do not pass it on to others.
It may harm them, even if their symptoms are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the leaflet. See section 4.
What is in this leaflet
1. What Pregnyl is and what it is used for
2. What you need to know before you take Pregnyl
3. How to use Pregnyl
4. Possible side effects
5. How to store Pregnyl
6. Contents of the pack and other information
1. What Pregnyl is and what it is used for
Pregnyl belongs to a group of medicines called gonadotrophins (sex hormones). It controls the release of eggs from the ovary in women, and controls production of the male hormone, testosterone in men. Women
In female infertility it can be used to cause women to ovulate (Ovulation induction). Pregnyl is also used along with other fertility drugs, to help produce eggs in medically assisted reproduction programmes. (IVF treatment).
Men
In men it is used to help treat delayed puberty, undescended testes or
oligospermia (low sperm count).
Ask your doctor if you are unsure why you have been given Pregnyl.
2. What you need to know before you take Pregnyl
Do not use Pregnyl if you:
• are allergic (hypersensitive) to Human Chorionic Gonadotrophin (HCG) or any of the other ingredients of this medicine (listed in section 6).
• have a thyroid, adrenal or pituitary illness which is not being treated
• have cancer, (especially a hormone-dependent cancer of the breast, ovaries or womb)
• have recently had unexpected vaginal bleeding
• if you have fibroids in the womb or abnormalities of the sexual organs which make a normal pregnancy impossible
• are a man and have, or suspect you have a hormone related tumour, such as testicular, prostate or breast cancer.
Warnings and precautions Medicines are not always suitable for everyone.
Talk to your doctor before using Pregnyl if you suffer from or have suffered in the past from any of the following conditions:
• men with
o heart problems o kidney problems o high blood pressure o epilepsy or o migraine
• abnormalities of the sexual organs. Before treatment with Pregnyl your doctor should have checked that your sexual organs are normal
• in women patients your doctor should have checked how your ovaries are working before starting treatment with Pregnyl. Extra supervision may be necessary in some cases.
Children and adolescents
Pregnyl should be used carefully when treating boys who have not reached puberty. This is because it can cause early sexual development and may result in final adult height not being reached.
Other Medicines and Pregnyl
Some medicines can affect the way Pregnyl works, or Pregnyl may affect how other medicines work. For the ten days following your Pregnyl injection a pregnancy test may give a false positive result.
* Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Fertility treatment
Close supervision of female patients undergoing fertility treatment is extremely important to avoid the rare complication of hyperstimulation of the ovaries.
This side effect may be felt as pain in the stomach.
* If you are troubled with stomach pains, contact your doctor straight away.
No one is sure if IVF treatment causes congenital malformations, or some cancers of the sex organs.
If you have risks factors for having a blood clot (for example being overweight, or if blood clots run in your family), the chance of having a blood clot may be increased during IVF treatment.
Being pregnant increases the chance of having a blood clot.
Pregnancy
If treatment with Pregnyl results in pregnancy, there is an increased chance having twins or multiple births. Multiple pregnancies carry an increased health risk for both the mother and her babies around the time of birth. There is also an increased chance of a miscarriage, or a pregnancy outside of the womb (an ectopic pregnancy).
3. How to use Pregnyl
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure.
Your doctor will choose the most suitable starting dose for you. The usual starting doses for men and women are as follows: Women
Patients undergoing ovulation induction:
5.000- 10,000 I.U. Pregnyl following treatment with other fertility drugs.
2 to 3 repeat injections of 1,000 to
3,000 I.U. each may be given within the following 9 days.
Patients undergoing IVF treatment:
5.000- 10,000 I.U. Pregnyl 30-40 hours after the last injection of other fertility drugs.
Men
In male patients injections are given 2 to 3 times a week for some weeks or months, depending on the problem. Because the development of sperm cells takes about 74 days, treatment should be continued for at least three months before any improvement can be expected.
How are the injections given?
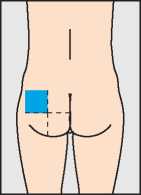
The very first injection of Pregnyl should only be given under medical supervision. Injections may be given slowly into a muscle (for instance in the bottom, upper leg or upper arm) or under the skin (in the stomach wall, for example). When given into a muscle the injection should be given by the doctor or nurse. The best site for injection of Pregnyl is the muscle of your bottom. The area shown in blue in the diagram contains a large amount of muscle with few blood vessels or major nerves.
When given under the skin the injection may, in some cases, be given by yourself or your partner. Your doctor will tell you when and how to do this. If you inject yourself with Pregnyl, follow the instructions on this leaflet carefully to give Pregnyl properly and with minimal discomfort.
Step 1 - Preparing Pregnyl
Pregnyl comes in two glass ampoules whose contents must be mixed together and used immediately.
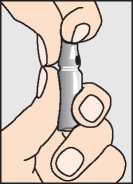
First, break the top off the ampoule with the sodium chloride solution
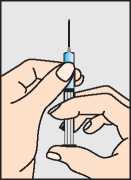
Draw up the liquid through the larger needle into the syringe (b).
b.
Break open the second ampoule containing the dry white powder (c) and add the sodium chloride solution from the syringe (d).
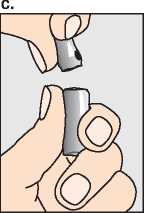
Do not shake, but gently swirl until the solution is clear.
The Pregnyl usually dissolves immediately. If the solution contains particles or does not become clear, do not use it.
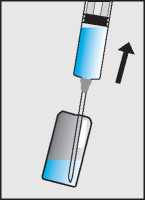
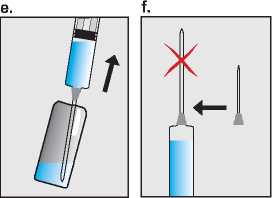
Draw the Pregnyl solution up into the empty syringe (e), and now replace the needle with a smaller sterile injection needle (f). Finally hold the syringe with the needle pointing upwards and gently tap the side to force any air bubbles up to the top; then squeeze the plunger until all the air has been expelled, and only Pregnyl solution is left in the syringe (g).
Bar prints 100mm @ 100%
|
= |
MSD ARTWORK |
|
= |
Item No.: 11022 Revision Date & No.: 150915-2 Pharma Code: 402 SchawkJobNo.: 487796A02 |
|
I |
Black Cyan Magenta Yellow |
|
= |
Technical Info Profile |
|
= |
SCHAWK! ■95% 5% ^^^^Proprietary |
In the male
Treatment with hCG leads to increased androgen production. Therefore:
■ Patients with latent or overt cardiac failure, renal dysfunction, hypertension, epilepsy or migraine (or a history of these conditions) should be kept under close medical supervision, since aggravation or recurrence may occasionally be induced as a result of increased androgen production.
■ HCG should be used cautiously in prepubertal boys to avoid premature epiphyseal closure or precocious sexual development. Skeletal maturation should be monitored regularly.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed; interactions with commonly used medicinal products can therefore not be excluded.
Following administration, Pregnyl may interfere for up to ten days with the immunological determination of serum/urinary hCG, leading to a false positive pregnancy test.
4.6 Fertility, pregnancy and lactation
Not applicable.
4.7 Effects on ability to drive and use machines
As far as known Pregnyl has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Frequency is unknown for all undesirable effects described below (cannot be determined with available data).
Immune system disorders
In rare cases generalized rash or fever may occur.
General disorders and administrative site conditions
Local site reactions such as bruising, pain, redness, swelling and itching. Oedema. Occasionally allergic reactions have been reported, mostly manifesting as pain and/or rash at the injection site. Tiredness.
Nervous system disorders Headache.
Psychiatric disorders
Mood changes.
In the female
Reproductive system and breast disorders
Unwanted ovarian hyperstimulation, mild or severe ovarian hyperstimulation syndrome
(OHSS, see section 4.4):
Mild OHSS:
Painful breasts
Mild to moderate enlargement of ovaries Ovarian cysts Abdominal pain Abdominal discomfort Gastrointestinal symptoms such as nausea, diarrhoea and bloating Severe OHSS:
Large ovarian cysts (prone to rupture),
Acute abdominal pain
Ascites
Weight gain
Hydrothorax
In rare instances, thromboembolism has been associated with FSH/hCG therapy
Not all symptoms described are always associated to OHSS.
In the male
Metabolism and nutrition disorders
Water and sodium retention is occasionally seen after administration of high dosages; this is regarded as a result of excessive androgen production.
Reproduction system and breast disorders
HCG treatment may sporadically cause gynaecomastia.
Skin and subcutaneous tissue disorders
Acne may occur occasionally during hCG therapy.
Reporting of suspect adverse reactions Reporting suspect adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspect adverse reactions via the Yellow Card Scheme, at www.mhra.gov.uk/yellowcard.
4.9 Overdose
The toxicity of human chorionic gonadotrophic hormone is very low. However, too high a dose may lead to hyperstimulation of the ovaries.
(See "Unwanted Hyperstimulation’).
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: gonadotrophins: ATC code G03G A01
Pregnyl is a preparation of human chorionic gonadotrophin obtained from the urine of pregnant women.
It stimulates the steroidogenesis in the gonads by virtue of a biological effect similar to that of LH (Luteinizing hormone, which is the same as interstitial cell stimulating hormone).
In the male it promotes the production of testosterone and in the female the production of estrogens and particularly of progesterone after ovulation. In certain cases, this preparation is used in combination with human menopausal gonadotrophin (HMG).
Because HCG is of human origin, no antibody formation is to be expected.
5.2 Pharmacokinetic properties
In a study performed in healthy male subjects, maximal hCG plasma levels were reached after a single IM or SC injection of hCG at approximately six and sixteen hours respectively; in addition, maximum concentrations and areas under the concentration curves were higher after the IM than after the SC injection. However, these differences did not translate into significant differences in terms of testicular steroidogenic response.
In a study performed in female subjects under oral contraceptives,
IM and SC administration of hCG were found to be bioequivalent regarding the extent of absorption and the apparent elimination half-lives of approximately 33 hours; maximal hCG plasma levels were reached after approximately 20 hours regardless of the route of administration.
Although high intersubject variability was observed, the difference related to gender after IM injection may be caused by gluteal fat thickness in women which exceeds that in men. In another study performed in female patients in the early follicular phase of their menstrual cycle, the bioavailability of a single dose of hCG was higher with the IM route than with the SC route and lower in obese women than in non-obese women.
11022/150915-2
HCG is approximately 80 per cent metabolized, predominantly in the kidneys.
On basis of the recommended dose regimens and elimination half-life, accumulation is not expected to occur.
5.3 Preclinical safety data
There are no preclinical data of relevance to the prescriber which are additional to that already included in other sections of the SPC.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients Powder for injection contains:
Carmellose sodium Mannitol (E421)
Disodium phosphate (anhydrous) Sodium dihydrogen phosphate (anhydrous)
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
36 months.
6.4 Special precautions for storage
Store in refrigerator (2°C to 8°C).
Do not freeze. Keep the ampoules in the outer carton to protect from light.
6.5 Nature and contents of container
2ml ampoule containing freeze-dried powder with 1ml ampoule of solvent (sodium chloride 9mg/ml).
Pregnyl is available in packs of 1, 3, 5 or 10 ampoules of powder and solvent.
Not all pack sizes may be marketed.
In correspondence please quote batch number.
6.6 Special precautions for disposal and other handling
Pregnyl should be reconstituted with the solvent provided. Do not use if the solution contains particles or if the solution is not clear. Since an opened ampoule cannot be resealed in such a way to further guarantee the sterility of the contents, the solution should be used immediately after reconstitution. Discard any remaining solution after single use.
Any unused product or waste material should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Merck Sharp & Dohme Limited
Hertford Road
Hoddesdon
Hertfordshire
EN11 9BU
UK
8. MARKETING AUTHORISATION NUMBER(S)
PL 00025/0555
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
24th February 1991/25th March 2003
10. DATE OF REVISION OF THE TEXT
July 2015
11. LEGAL CATEGORY
Prescription Only Medicine SPC.PRG.1500.14.UK.4427-IB-QRD © Merck Sharp & Dohme Limited 2015. All rights reserved.


9-
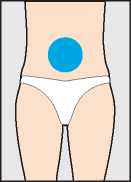
Step 2 - The injection site
The best site for injection is in the stomach around the middle of the tummy (h) where there is a lot of loose skin and layers of fatty tissue. Pinch up a large area of skin between the finger and thumb.
You should change the injection site a little each time you inject. It is possible to inject in other areas. Your doctor or nurse will advise you where to inject.
Step 3 - Preparing the area A few taps at the injection site will stimulate tiny nerve endings and help reduce discomfort when the needle goes in. Hands should be washed and the injection site swabbed with disinfectant (for example chlorohexidine 0.5%) to remove any surface bacteria. Clean about two inches around the point where the needle will go in and let the disinfectant dry for at least one minute before proceeding.
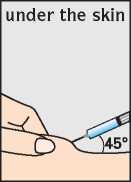
Step 4 - Inserting the needle
The needle should be inserted at the base of the pinched-up skin at an angle of 45° to the skin surface (i).
Step 5 - Checking the correct needle position
If the needle position is correct the plunger should be quite difficult to draw back. Any blood sucked back into the syringe means that the needle tip has entered a vein or artery. If this happens pull out the syringe, cover the injection site with a swab containing disinfectant and apply pressure; the site will stop bleeding in a minute or two. Do not use this solution but flush it away.
Start again with Step 1 using a new needle and new ampoules of Pregnyl and sodium chloride solution.
Step 6 - Injecting the solution
Depress the syringe plunger slowly and steadily, so the solution is correctly injected and
the muscle or skin tissues are not damaged.
Step 7 - Removing the syringe
Pull the syringe out quickly and apply pressure to the injection site with a swab containing disinfectant. A gentle massage of the site - while still maintaining pressure -helps disperse the Pregnyl solution and relieve any discomfort. Any remaining solution should be discarded. Do not mix Pregnyl solution with any other medicines.
Step 8 - Disposing of needles
Replace the needle guard on the syringe to prevent injury.
Carefully dispose of any needles that you use.
You can dispose of needles in a 'sharps bin1, or take them to your local pharmacy for disposal. Do not share your needles or syringes.
Always take Pregnyl exactly as your doctor has told you. Check with your doctor or pharmacist if you are still not sure.
If you take more Pregnyl than you should
As your doctor will be keeping a close eye on you it is unlikely you will be given too much, however too high a dose of Pregnyl may cause hyperstimulation of the ovaries. This may be noticed as pain in the abdomen. See section on Possible side effects below. If you are troubled by stomach pains, tell your doctor immediately.
If you accidentally use too much
Pregnyl contact your doctor at once or go to the nearest hospital casualty department. Always take the labelled medicine package with you, whether there is any Pregnyl left or not.
If you forget to take Pregnyl If you forget to take a dose do not take a double dose to make up for a missed dose.
* Contact your doctor.
If you stop taking Pregnyl
Do not stop taking Pregnyl unless your doctor tells you to. Your doctor will advise you if you need to stop using Pregnyl for any reason.
If you have any further questions on how to take Pregnyl, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects: tell a doctor straight away
If you have an allergic reaction to Pregnyl see a doctor straight away
• Pregnyl may cause reactions at the site of injection, such as bruising, pain, redness, swelling and rashes at the injection site.
• more widespread rash and fever may occur.
Contact a doctor immediately if you are a woman and experience:
Severe pain in the abdomen, feeling sick (nausea), diarrhoea, painful breasts, also if it occurs a few days after you receive your last injection, since it could be a sign of unwanted overstimulation of the ovaries (OHSS).
If you are a woman:
If your ovaries have been excessively stimulated by an FSH-containing preparation and Pregnyl is given, it may lead to unwanted overstimulation of the ovaries. This condition (also called OHSS) can become very serious, but the risk can be minimised by careful monitoring of egg cell development during treatment. The first symptoms of ovarian overstimulation may be noticed as pain in the stomach (abdomen), feeling sick (nausea) or diarrhoea. In more severe cases symptoms may include enlargement of the ovaries, accumulation of fluid in the abdomen and/or chest, weight gain and the occurrence of blood clots in the circulation.
* Contact your doctor without delay if you are experiencing significant abdominal pain, also if this occurs some days after the last injection has been given. The following side effects might be the result of OHSS: pain in the stomach (abdomen) feeling sick (nausea) diarrhoea bloating
ovarian cysts or enlargement of the ovaries
painful breasts palpable ovarian cysts accumulation of fluid in the abdomen and/ or chest blood clots
ovarian cysts prone to rupture weight gain
If you are a man:
fluid may be retained in the tissues, usually marked by swelling of ankles or feet, and occasionally enlargement of the breast may occur. This can be caused by an increased androgen production by treatment with hCG.
* If any of these signs appear, tell your doctor immediately.
Other possible side effects
acne (in men) fluid retention headache tiredness mood changes
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Pregnyl
Keep out of the sight and reach of children.
Pregnyl should be stored in a refrigerator (2°C to 8°C).
Do not freeze.
Keep the ampoules in the outer carton in order to protect from light.
Do not use Pregnyl after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to throw away medicines you no longer use.
These measures will help to protect the environment.
6. Contents of pack and other information
What Pregnyl contains
Each ampoule contains 1500 I.U. of the active ingredient Human Chorionic Gonadotrophin.
The other ingredients are carmellose sodium, mannitol (E421), disodium phosphate (anhydrous), sodium dihydrogen phosphate (anhydrous). The solvent contains sodium chloride (9 mg) and water for injections.
What Pregnyl looks like and contents of the pack
Pregnyl comes as 2 ml ampoules of dry white powder with 1 ml ampoule of solvent (sodium chloride solution). Pregnyl 1500 I.U. is available in packs of 1, 3, 5 or 10 ampoules of powder and solvent. Not all pack sizes may be marketed.
Market Authorisation Holder and Manufacturer
Marketing Authorisation Holder
Merck Sharp & Dohme Limited,
Hertford Road, Hoddesdon,
Hertfordshire, EN11 9BU, UK Manufacturer
N.V Organon, Oss, The Netherlands.
This leaflet was last updated in July 2015
Other sources of information
In correspondence please quote packing number.
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge: 0800 198 5000 (UK Only) Please be ready to give the following information:
Product name: Pregnyl 1500 I.U. Reference Number: PL 00025/0555 This is a service provided by the Royal National Institute of Blind people. PIL.PRG. 1500.14.UK.4428 IB-QRD © Merck Sharp & Dohme limited, 2015. All rights reserved.
Over Sticker
Varnish Free
Text Free
Pharma Code
Braille Grid
Tamper Evident
Profile Perforation Fold