Primolut N
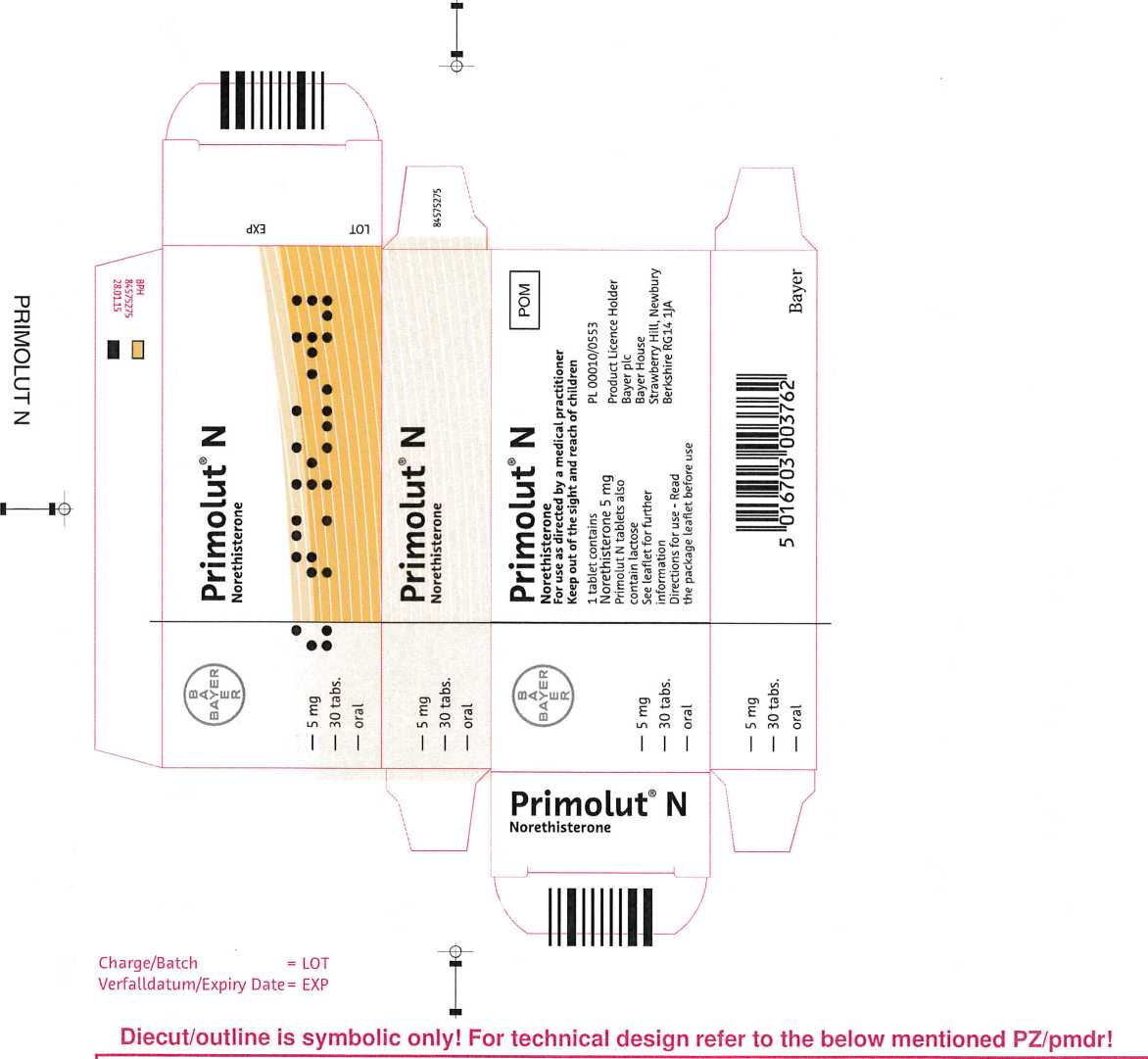
Bayer Pharma AG - Packaging Technology Berlin
PZ/pmdr.:
Code-Nr./code-no.:
Bezeichnung/name: 1666AB-4 1795
Primolut N TAB 3x10
Stoffnr,/item-no:
Mandant/client:
Aufmachung/country:
84575275
Weimar
GB/-/BPH
Prageplatte/embossing plate:
Abmessungen/Dimension:
36.0x18.0x85.0 mm
Diecut/outline is symbolic only! For technical design refer to the below mentioned PZ/pmdr!
|
Bayer Pharma AG - Packaging Technology Berlin | |
|
PZ/pmdr.: 1666AB-4 Stoffnr./item-no: |
84575275 |
|
Code-Nr./code-no.: 1795 Mandant/client: |
Weimar |
|
Bezeichnunq/name: Primolut N TAB 3x10 Aufmachunq/country: |
GB/-/BPH |
|
Prageplatte/embossing plate: Abmessungen/Dimension: |
36.0x18.0x85.0 mm |
|
ETokKoi io"7i !nr\\r\r ccmaratirsn- | |

Package leaflet: Information for the user
bayer! Primolut® N
IE/
R y 5 mg tablets
(norethisterone)
Read all of this Leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet You may need to read it again.
If you have any further questions, ask your doctor or pharmacist
This medicine has been prescribed for you only. Do not pass it on to others. It
may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Primolut N Is and what it is used for
2. What you need to know before you take Primolut N
3. How to take Primolut N
4. Possible side effects
5. How to store Primolut N
6. Contents of the pack and other information
1. What Primolut N is and what it is used for
Primolut N contains norethisterone, which belongs to a group of medicines called progestagens, which are female hormones.
Primolut N can be used in several different Circumstances:
► to treat irregular, painful or heavy periods
► to treat endometriosis (where tissue from the Lining of the womb is present in places where it is not normally found)
► to treat premenstrual syndrome (also known as premenstrual tension, PMS or PMT)
► to delay periods
2. What you need to know before you take Primolut N
► Your doctor will discuss your medical and family history with you. Your doctor will also need to check your blood pressure and make sure you are not pregnant You may also need additional checks, such as a breast examination, that will be specific to your medical needs and/or concerns.
Do not take Primolut N:
► if you are allergic to norethisterone or any of the other ingredients of this medicine (listed in section 6)
► if you are pregnant or if you think you might be pregnant
► if you are breast-feeding
or:
► if you have ever had a problem with your blood circulation. This includes a blood clot (thrombosis) in the legs (deep vein thrombosis), lungs (pulmonary embolism), heart (heart attack), brain (stroke) or any other parts of the body
► if you have any symptoms of a blood clot, such as chest pain, unexplained and often sudden shortness of breath and/or cough
► if you have any condition which makes you more at risk of a blood clot (thrombosis)
► if you have ever suffered migraine with visual disturbance
► if you have (or are recovering from) a liver disease and the blood tests show that your liver is not yet working normally
► if you have (or have ever had) liver tumours
► if you have diabetes with damaged blood vessels
► if you have any type of cancer which might be made worse by exposure to female sex hormones (including breast cancer)
► if you have problems with genital bleeding for which the cause is not yet known
► if you have a condition called endometrial hyperplasia which has not been treated.
In addition, do not take Primolut N if you have had any of the following conditions when you
were pregnant;
► yellowing of the skin (idiopathic jaundice of pregnancy)
► itching of the whole body (pruritus of pregnancy)
-*• Tell your doctor if any of these apply to you and do not take Primolut N.
Warnings and precautions
Talk to your doctor or pharmacist before taking Primolut N
► ifyou smoke
► ifyou have diabetes. Primolut N can produce changes in blood sugar levels. Ifyou are diabetic, your doctor will check your blood sugar before starting treatment and regularly during treatment
► ifyou are overweight (BMI130 kg/m!)
► ifyou have high blood pressure
► ifyou have a heart valve disorder or a certain heart rhythm disorder (heart problems)
► ifyou have had a thrombosis/embolism or anyone in your close family has had a thrombosis, a heart attack or a stroke at a young age
► if you suffer from migraine, asthma, or kidney problems
► if you suffer from epilepsy (see "Other medicines and Primolut N")
► if you have an inflammation of your veins (superficial phlebitis)
► if you have varicose veins
► if anyone in your immediate family has had breast cancer
► if you have previously had a condition called chloasma where the skin on your face may develop brownish blotches. You may be advised to avoid exposure to the sun and to ultraviolet light while you are taking Primolut N
► if you have previously suffered from depression
► if you or someone in your close family has ever had high blood levels of cholesterol or triglycerides (fatty substances)
► ifyou have a disease ofthe liver orgall bladder
► if you have certain rare medical conditions such as systemic lupus erythematosus (SLE), sickle cell disease, Crohn’s disease or ulcerative colitis
► ifyou have haemolytic uremic syndrome (HUS)
► if you have a condition that occurred for the first time or worsened during pregnancy or previous use of sex hormones (e.g. hearing loss, porphyria, or Sydenham’s chorea)
► ifyou have hereditary angioedema. Consult your doctor immediately ifyou experience symptoms of angioedema such as swollen face, tongue or throat, and/or difficulty swallowing, or hives, together with difficulty breathing. Products containing oestrogens may induce or worsen symptoms of angioedema
► ifyou have an intolerance to some types of sugar (galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption)
► if you are using other medications as mentioned in "Other Medicines and Primolut N”.
Tell your doctor before you take Primolut N if any of these applies to you. Also tell your doctor if any of these conditions develop or worsen while you are taking Primolut N, because you may need to stop taking it
Primolut N and blood clots:
The main ingredient in Primolut N (progestogen) is partly converted into oestrogen so you should also consider the general warnings given for combined oral contraceptive pills ("the Pill").
Do not take Primolut N ifyou have a blood clot or have any medical condition which makes you more at risk of developing clots.
The risk of blood clots occurring in the veins and arteries is slightly greater in women who take the combined oral contraceptive pill than in women who don’t. People do not always fully recover from such blood Clots, which can cause strokes, heart attacks and bleeding into the brain (subarachnoid haemorrhage). In very rare cases these blood clots can be fatal.
You are more at risk of having a blood clot:
► as you get older
► if you're off your feet for a long time because of major surgery, injury or illness.
► ifyou smoke
► ifyou or any of your close family have had blood clots
► ifyou are overweight (BMI > 30 kg/mJ)
► ifyou have a disorder of blood fat (lipid) metabolism
► ifyou have a blood disorder
► if you have high blood pressure
► if you suffer from migraines
► ifyou have a heart valve disorder or a particular type of irregular heartbeat (atrial fibrillation)
► ifyou have recently had a baby
► if you have diabetes
► ifyou have certain medical conditions such as systemic lupus erythematosus (SLE), sickle cell disease, Crohn's disease or ulcerative colitis
-► Tell your doctor if any of these apply to you. Taking Primolut N may add to this risk SO it may not be suitable for you.
To reduce the risk of blood clots, treatment with Primolut N must be stopped;
► six weeks before any planned major operation
► before any surgery to the legs
► before medical treatment for varicose veins
► ifyou are going to be immobilised for a long time (e.g. ifyou need bed-rest after an accident or operation, or if you have a plaster cast on a broken leg)
Signs of a blood clot include:
► a migraine for the first time or one that is worse than normal
► unusually freguent or severe headaches
► any sudden changes to your eyesight (such as loss of vision or blurred vision)
► any sudden changes to your hearing, speech, sense of smell, taste or touch
► pain or swelling in your leg
► stabbing pain when you breathe
► coughing for no apparent reason
► breathlessness
► pain and tightness in the chest
► sudden weakness or numbness in one side or part of your body
► dizziness or fainting.
-> See a doctor as soon as possible if you notice any possible signs of blood clot. Do not take any more Primolut N until your doctor says you can.
Primolut N and cancer
Ifyou have breast cancer, or have had it in the past, you should not take combined oral contraceptives (the Pill). The Pill slightly increases your risk of breast cancer. This risk goes up the longer you're on it, but returns to normal within about 10 years of stopping it. Because breast cancer is rare in women under the age of <*0, the extra cases of breast cancer in current and recent Pill users is small. For example:
► Of 10,000 women who have never taken the Pill, about 16 will have breast cancer by the time they are 35 years old.
Please turn over
i
l
%
► Of 10,000 women who take the Pill for 5 years in their early twenties, about 17-18 will have breast cancer by the time they are 35 years old.
► Of 10,000 women who have never taken the Pill, about 100 will have breast cancer by the time they are 45 years old.
► Of 10,000 women who take the Pill for 5 years in their early thirties, about 110 will have breast cancer by the time they are 45 years old.
Your risk of breast cancer is higher if:
► you have a close relative (mother, sister or grandmother) who has had breast cancer
► you are overweight (BMI £ 30 kg/m1)
-*• See a doctor as soon as possible if you notice any changes in your breasts, such as dimpling of the skin, changes in the nipple or any lumps you can see or feel.
Very rarely, the Pill has been linked with some forms of liver cancer in women who take it for a long time. These may lead to bleeding in the abdomen.
Taking the Pill has also been linked to liver diseases, such as jaundice and non-cancerous liver tumours, but this is rare.
See a doctor as soon as possible if you get severe pain in your stomach that does not go away, or yellow skin or eyes (jaundice). You may need to stop taking Primolut N. Other medicines and Primolut N
-► Tell your doctor if you are taking, have recently taken or might take any other medicines. Some medicines may affect the way Primolut N works. Tell your doctor if you are taking:
► Phenytoin, oxcarbazepine, primidone or carbamazepine. These drugs are often used to treat epilepsy.
► Sedative drugs called barbiturates.
► The antibiotic drugs rifampicin or rifabutin.
► An antifungal drug called griseofulvin.
► St John’s wort to treat depression.
Some other medicines may be affected by Primolut N. Tell your doctor if you are taking a drug called ciclosporin which is often used after an organ transplant.
Taking Primolut N can affect the results of some blood and urine tests. Tell your doctor that you are taking Primolut N if you are asked to provide a blood or urine sample.
Other things you should know:
Once you have finished taking a course of Primolut N, you will usually have a menstrual bleed (period) 2*3 days after taking your last tablet. If you do not have a period, you must make sure that you are not pregnant before taking any more tablets.
Pregnancy and breast-feeding
Do not take Primolut N if you are pregnant or breast-feeding. If you think you may be
pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Primolut N is unlikely to affect your ability to drive or use machines.
Primolut N contains lactose
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take Primolut N
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The number of tablets that you need to take and the number of days per month when you need to take them will depend on why the doctor has prescribed Primolut N. A common dosage would be 2-3 tablets each day. For some conditions Primolut N has to be taken every day, but this is not always the case.
Ask your doctor or pharmacist, if you are not sure about the number of tablets that you need to take, when they should be taken or how long you should take them for.
Swallow the tablets whole with a drink of water.
If you take more Primolut N than you should
Taking too many tablets is unlikely to cause serious problems. If you take too many, contact your doctor who will tell you what to do.
If you forget to take Primolut N
If you forget a dose, wait until it is time to take the next prescribed dose. Do not take the missed dose. If you are worried, contact your doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Reasons for stopping Primolut N immediately:
Stop taking Primolut N and speak to your doctor immediately if you experience any of the following:
► migraine for the first time
► unusually bad headaches, occurring more often than before
► sudden changes to your eyesight, hearing or speech
► sudden changes to your senses of smell, taste or touch
► symptoms of blood clot formation or symptoms of inflammation of the veins combined with the formation of blood clots (thrombophlebitis):
> unusual pains in your leg(s)
> unusual swelling of your arms or legs
> sharp pains in your chest or sudden shortness of breath
> crushing pains or feelings of heaviness or tightness in your chest
> coughing for no apparent reason
> one side of your body suddenly becoming very weak or numb
Primolut N must also be stopped immediately if:
► you become pregnant
► you develop jaundice or other liver problems
► you develop itching (pruritus)
► your doctor finds that your blood pressure is too high General side effects:
Side effects that have been reported with Primolut N are listed below according to the frequency with which they occur.
Very common side effects
(These may affect more than 1 in 10 people)
► Vaginal bleeding, including spotting.
► Periods that are much shorter than normal and where blood flow is reduced. Common side effects
(These may affect up to 1 in 10 people)
► Headache
► Feeling sick (nausea)
► Absence of a period
► Swelling
Uncommon side effects
(These may affect up to 1 in 100 people)
► Migraine Rare side effects
(These may affect up to 1 in 1,000 people)
► Allergic reactions including skin rash or "hives"
Very rare side effects
(These may affect up to 1 in 10,000 people)
► Visual disturbances
► Difficulty in breathing Other side effects
(Frequency not known: frequency cannot be estimated from the available data)
► Worsening of depression
► Dizziness
► Abdominal pain
► Jaundice
► Cholestasis
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist This includes any possible side
effects not listed in this leaflet. You can also report side effects directly (see details below). By
reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Malta
ADR Reporting
Website: www.medicinesauthority.gov.mt/adrportal
5. How to store Primolut N
Keep this medicine out of the sight and reach of children.
Store in the original carton.
Do not use this medicine after the expiry date which is stated on both the outer carton and on each blister strip of tablets after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Primolut N contains
Each tablet contains 5 mg of the active ingredient, norethisterone. The other ingredients are lactose, maize starch and magnesium stearate (E572).
What Primolut N looks like and contents of the pack
Each pack contains 30 tablets (3 foil blister packs containing 10 tablets each). Each white tablet has 'AN' embossed in a regular hexagon on one side.
Marketing Authorisation Holder and Manufacturer Marketing authorisation holder:
Bayer pic Bayer House Strawberry Hill Newbury Berkshire RG14 1JA United Kingdom Manufacturers:
Bayer Pharma AG, Berlin, Germany or
Bayer Weimar GmbH & Co. KG, 99427 Weimar, Germany This leaflet was last revised in May 2015.
Product licence number: ^
pl 00010/0553 bayer
84575283

|
Packaqinq Technology Berlin somol |
Bayer Pharma AG | |
|
client: IS86 item-no : 84S7S283 |
PZ: 2S98F-4 code-no : 179S | |
|
name: IF-Primolut N SMG TAB | ||
|
colors: Black | ||
|
version: 19.0S.201S/02 |
approval: |
dimension: 160 x 420 mm |

Package leaflet: Information for the user
Primolut® N
5 mg tablets (norethisterone)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others, it may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talkto your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Primolut N is and what it is used for
2. What you need to know before you take Primolut N
3. How to take Primolut N
4. Possible side effects
5. How to store Primolut N
6. Contents of the pack and other information
1. What Primolut N is and what it is used for
Primolut N contains norethisterone, which belongs to a group of medicines called progestogens, which are female hormones.
Primolut N can be used in several different circumstances:
► to treat irregular, painful or heavy periods
► to treat endometriosis (where tissue from the lining of the womb is present in places where it is not normally found)
► to treat premenstrual syndrome (also known as premenstrual tension, PMS or PMT)
► to delay periods
2. What you need to know before you take Primolut N
► Your doctor will discuss your medical and family history with you. Your doctor will also need to check your blood pressure and make sure you are not pregnant. You may also need additional checks, such as a breast examination, that will be specific to your medica needs and/or concerns.
Do not take Primolut N:
► if you are allergic to norethisterone or any of the other ingredients of this medicine (listed in section 6)
► if you are pregnant or if you think you might be pregnant
► if you are breast-feeding
or:
► if you have ever had a problem with your blood circulation. This includes a blood clot
(■thrombosis) in the legs (deep vein thrombosis), lungs {pulmonary embolism), heart (heart attack), brain (stroke) or any other parts of the body
► if you have any symptoms of a blood clot, such as chest pain, unexplained and often sudden shortness of breath and/or cough
► if you have any condition which makes you more at risk of a blood clot (thrombosis)
► if you have ever suffered migraine with visual disturbance
► if you have (or are recovering from) a liver disease and the blood tests show that your liver is not yet working normally
► if you have (or have ever had) liver tumours
► if you have diabetes with damaged blood vessels
► if you have any type of cancer which might be made worse by exposure to female sex hormones (including breast cancer)
► if you have problems with genital bleeding for which the cause is not yet known
► if you have a condition called endometrial hyperplasia which has not been treated.
In addition, do not take Primolut N if you have had any of the following conditions when you
were pregnant:
► yellowing of the skin (idiopathic jaundice of pregnancy)
► itching of the whole body (pruritus of pregnancy)
-► Tell your doctor if any of these apply to you and do not take Primolut N.
Warnings and precautions
Talkto your doctor or pharmacist before taking Primolut N
► if you smoke
► if you have diabetes. Primolut N can produce changes in blood sugar levels, if you are diabetic, your doctor will check your blood sugar before starting treatment and regularly during treatment
► if you are overweight (BMI > 30 kg/m2)
► if you have high blood pressure
► if you have a heart valve disorder or a certain heart rhythm disorder (heart problems)
► if you have had a thrombosis/embolism or anyone in your close family has had a thrombosis, a heart attack or a stroke at a young age
► if you suffer from migraine, asthma, or kidney problems
► if you suffer from epilepsy (see “Other medicines and Primolut N”)
► if you have an inflammation of your veins (superficial phlebitis)
► if you have varicose veins
► if anyone in your immediate family has had breast cancer
► if you have previously had a condition called chloasma where the skin on your face may develop brownish blotches. You may be advised to avoid exposure to the sun and to ultraviolet light while you are taking Primolut N
► if you have previously suffered from depression
► if you or someone in your close family has ever had high blood levels of cholesterol or triglycerides (fatty substances)
► if you have a disease of the liver or gall bladder
► if you have certain rare medical conditions such as systemic lupus erythematosus (SLE), sickle cell disease, Crohn’s disease or ulcerative colitis
► if you have haemolytic uremic syndrome (HUS)
► if you have a condition that occurred for the first time or worsened during pregnancy or previous use of sex hormones (e.g. hearing loss, porphyria, or Sydenham's chorea)
► if you have hereditary angioedema. Consult your doctor immediately if you experience symptoms of angioedema such as swollen face, tongue or throat, and/or difficulty swallowing, or hives, together with difficulty breathing. Products containing oestrogens may induce or worsen symptoms of angioedema
► if you have an intolerance to some types of sugar (galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption)
► if you are using other medications as mentioned in “Other Medicines and Primolut N”.
-► Tell your doctor before you take Primolut N if any of these applies to you. Also tell your
doctor if any of these conditions develop or worsen while you are taking Primolut N, because you may need to stop taking it.
Primolut N and blood clots:
The main ingredient in Primolut N (progestogen) is partly converted into oestrogen so you should also considerthe general warnings given for combined oral contraceptive pills (“the Pill”).
Do not take Primolut N if you have a blood clot or have any medical condition which makes you more at risk of developing clots.
The risk of blood clots occurring in the veins and arteries is slightly greater in women who take the combined oral contraceptive pill than in women who don’t. People do not always fully recover from such blood clots, which can cause strokes, heart attacks and bleeding into the brain [subarachnoidhaemorrhage). In very rare cases these blood clots can be fatal.
You are more at risk of having a blood clot:
► as you get older
► if you’re off your feet for a long time because of major surgery, injury or illness.
► if you smoke
► if you or any of your close family have had blood clots
► if you are overweight (BMI > 30 kg/m2)
► if you have a disorder of blood fat (lipid) metabolism
► if you have a blood disorder
► if you have high blood pressure
► if you suffer from migraines
► if you have a heart valve disorder or a particular type of irregular heartbeat (atrial fibrillation)
► if you have recently had a baby
► if you have diabetes
► if you have certain medical conditions such as systemic lupus erythematosus (SLE), sickle cell disease, Crohn’s disease or ulcerative colitis
-► Tell your doctor if any of these apply to you. Taking Primolut N may add to this risk so it may not be suitable for you.
To reduce the risk of blood clots, treatment with Primolut N must be stopped:
► six weeks before any planned major operation
► before any surgery to the legs
► before medical treatment for varicose veins
► if you are going to be immobilised for a long time (e.g. if you need bed-rest after an accident or operation, or if you have a plaster cast on a broken leg)
Signs of a blood clot include:
► a migraine for the first time or one that is worse than normal
► unusually frequent or severe headaches
► any sudden changes to your eyesight (such as loss of vision or blurred vision)
► any sudden changes to your hearing, speech, sense of smell, taste or touch
► pain or swelling in your leg
► stabbing pain when you breathe
► coughing for no apparent reason
► breathlessness
► pain and tightness in the chest
► sudden weakness or numbness in one side or part of your body
► dizziness orfainting.
-► See a doctor as soon as possible if you notice any possible signs of blood clot. Do not
take any more Primolut N until your doctor says you can.
Primolut N and cancer
if you have breast cancer, or have had it in the past, you should not take combined oral contraceptives (the Pill). The Pill slightly increases your risk of breast cancer. This risk goes up the longeryou’re on it, but returns to normal within about 10 years of stopping it. Because breast cancer is rare in women under the age of 40, the extra cases of breast cancer in current and recent Pill users is small. For example:
► Of 10,000 women who have never taken the Pill, about 16 will have breast cancer by the time they are 35 years old.
-► Please turn over
|
Packaqinq Technoloqv Berlin sqmql |
paqe 1 |
Baver Pharma AG |
|
client: IS86 item-no.: 84575283 |
PZ: 2598F-4 code-no.: 1795 | |
|
name: LF-Primolut N 5MGTAB |
country: GB/-/BPH | |
|
colors: Black | ||
|
version: 19.05.2015/02 |
approval: |
dimension: 150 x 420 mm |
► Of 10,000 women who take the Pill for 5 years in their early twenties, about 17-18 will have breast cancer by the time they are 35 years old.
► Of 10,000 women who have never taken the Pill, about 100 will have breast cancer by the time they are 45 years old.
► Of 10,000 women who take the Pill for 5 years in their early thirties, about 110 will have breast cancer by the time they are 45 years old.
Your risk of breast cancer is higher if:
► you have a close relative (mother, sister or grandmother) who has had breast cancer
► you are overweight (BMI > 30 kg/m2)
-► See a doctor as soon as possible if you notice any changes in your breasts, such as dimpling of the skin, changes in the nipple or any lumps you can see or feel.
Very rarely, the Pill has been linked with some forms of liver cancer in women who take it for a long time. These may lead to bleeding in the abdomen.
Taking the Pill has also been linked to liver diseases, such as jaundice and non-cancerous livertumours, but this is rare.
-► See a doctor as soon as possible if you get severe pain in your stomach that does not go away, or yellow skin or eyes (jaundice). You may need to stop taking Primolut N. Other medicines and Primolut N
-► Tell your doctor if you are taking, have recently taken or might take any other medicines. Some medicines may affect the way Primolut N works. Tell your doctor if you are taking:
► Phenytoin, oxcarbazepine, primidone or carbamazepine. These drugs are often used to treat epilepsy.
► Sedative drugs called barbiturates.
► The antibiotic drugs rifampicin or rifabutin.
► An antifungal drug called griseofulvin.
► St John’s wort to treat depression.
Some other medicines may be affected by Primolut N. Tell your doctor if you are taking a drug called ciclosporin which is often used after an organ transplant.
Taking Primolut N can affect the results of some blood and urine tests. Tell your doctor that you are taking Primolut N if you are asked to provide a blood or urine sample.
Other things you should know:
Once you have finished taking a course of Primolut N, you will usually have a menstrual bleed (period) 2-3 days after taking your last tablet. If you do not have a period, you must make sure that you are not pregnant before taking any more tablets.
Pregnancy and breast-feeding
Do not take Primolut N if you are pregnant or breast-feeding, if you think you may be
pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Primolut N is unlikely to affect your ability to drive or use machines.
Primolut N contains lactose
if you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take Primolut N
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The number of tablets that you need to take and the number of days per month when you need to take them will depend on why the doctor has prescribed Primolut N. A common dosage would be 2-3 tablets each day. For some conditions Primolut N has to be taken every day, but this is not always the case.
Ask your doctor or pharmacist, if you are not sure about the number of tablets that you need to take, when they should be taken or how long you should take them for.
Swallow the tablets whole with a drink of water.
If you take more Primolut N than you should
Taking too many tablets is unlikely to cause serious problems, if you take too many, contact your doctor who will tell you what to do.
If you forget to take Primolut N
If you forget a dose, wait until it is time to take the next prescribed dose. Do not take the missed dose. If you are worried, contact your doctor or pharmacist.
If you have any further questions on the use of this medicine, askyour doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Reasons for stopping Primolut N immediately:
Stop taking Primolut N and speakto your doctor immediately if you experience any of the following:
► migraine forthe first time
► unusually bad headaches, occurring more often than before
► sudden changes to your eyesight, hearing or speech
► sudden changes to your senses of smell, taste or touch
► symptoms of blood clot formation or symptoms of inflammation of the veins combined with the formation of blood clots (thrombophlebitis):
> unusual pains in your leg(s)
> unusual swelling of your arms or legs
> sharp pains in your chest or sudden shortness of breath
> crushing pains orfeelings of heaviness ortightness in your chest [> coughing for no apparent reason
> one side of your body suddenly becoming very weak or numb
Primolut N must also be stopped immediately if:
► you become pregnant
► you develop jaundice or other liver problems
► you develop itching (pruritus)
► your doctor finds that your blood pressure is too high General side effects:
Side effects that have been reported with Primolut N are listed below according to the frequency with which they occur.
Very common side effects
(These may affect more than 1 in 10 people)
► Vaginal bleeding, including spotting.
► Periods that are much shorterthan normal and where blood flow is reduced. Common side effects
(These may affect up to 1 in 10 people)
► Headache
► Feeling sick (nausea)
► Absence of a period
► Swelling
Uncommon side effects
(These may affect up to 1 in 100 people)
► Migraine Rare side effects
(These may affect up to 1 in 1,000 people)
► Allergic reactions including skin rash or “hives”
Very rare side effects
(These may affect up to 1 in 10,000 people)
► Visual disturbances
► Difficulty in breathing Other side effects
(Frequency not known: frequency cannot be estimated from the available data)
► Worsening of depression
► Dizziness
► Abdominal pain
► Jaundice
► Cholestasis
Reporting of side effects
If you get any side effects, talkto your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. You can also report side effects directly (see details below). By
reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Malta
ADR Reporting
Website: www.medicinesauthority.gov.mt/adrportal
5. How to store Primolut N
Keep this medicine out of the sight and reach of children.
Store in the original carton.
Do not use this medicine afterthe expiry date which is stated on both the outer carton and on each blister strip of tablets after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Askyour pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Primolut N contains
Each tablet contains 5 mg of the active ingredient, norethisterone. The other ingredients are lactose, maize starch and magnesium stearate (E572).
What Primolut N looks like and contents of the pack
Each pack contains 30 tablets (3 foil blister packs containing 10 tablets each). Each white tablet has ‘AN’ embossed in a regular hexagon on one side.
Marketing Authorisation Holder and Manufacturer Marketing authorisation holder:
Bayer pic Bayer House Strawberry Hill Newbury Berkshire RG14 1JA United Kingdom Manufacturers:
Bayer Pharma AG, Berlin, Germany or
Bayer Weimar GmbH &. Co. KG, 99427 Weimar, Germany This leaflet was last revised in May 2015.
Product licence number: ^
pl 00010/0553 r>ayer
84575283

|
Packaqinq TechnoLoqv Berlin sqmql paqe 2 |
Baver Pharma AG |
|
client: IS86 item-no.: 84575283 PZ: 2598F-4 |
code-no.: 1795 |
|
name: LF-PrimoLut N 5MGTAB |
country: GB/-/BPH |
|
colors: Black | |
version: 19.05.2015/02
approval.:
dimension: 160 x 420 mm