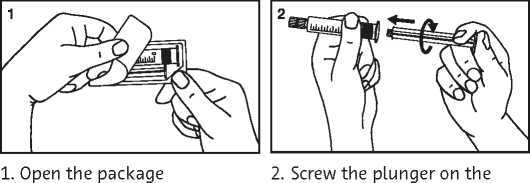
Primovist 0.25 Mmol/Ml Solution For Injection Prefilled Syringe
0

Package leaflet: Information for the user
Primovist 0.25 mmol/ml. solution for injection, pre-filled syringe
Gadoxetate, disodium
Read all of this leaflet carefully before you are given this medicine because it contains important information for you. R Keep this leaflet. You may need to read it again.
R If you have any further questions, please ask your doctor giving you Primovist (the radiologist) or the hospital/ MRI-centre personnel.
R If you get any side effects talk to your doctor or radiologist. This includes any possible side effects not listed in this leaflet. See section 4.
|
What is in this leaflet | |
|
1. |
What Primovist is and what it is used for |
|
2. |
What you need to know before you are given Primovist |
|
3. |
How to use Primovist |
|
4. |
Possible side effects |
|
5. |
How to store Primovist |
|
6. |
Contents of the pack and other information |
1. What Primovist is and what it is used for
Primovist is a contrast medium for magnetic resonance imaging (MRI) of the liver. It is used to help detect and diagnose changes that may be found in the liver. Abnormal signs within the liver can be better evaluated (as to number, size, and distribution). Primovist can also help the doctor determine the nature of any abnormalities, thereby increasing the confidence one can have in the diagnosis.
It is provided as a solution for intravenous injection. This medicine is for diagnostic use only.
MRI is a form of medical diagnostic imaging that forms pictures after water molecules have been detected in normal and abnormal tissues. This is done using a complex system of magnets and radiowaves.
2. What you need to know before you are given Primovist Do not use Primovist
R if you are allergic to gadoxetate disodium or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you are given Primovist if you R have or have had allergy (e.g. hay fever, hives) or asthma R had a previous reaction to contrast media R have a poor kidney function.
The use of some gadolinium-containing contrast agents in patients with these conditions has been associated with a disease called Nephrogenic Systemic Fibrosis (NSF). NSF is a disease involving thickening of the skin and connective tissues. NSF may result in debilitating joint immobility, muscle weakness or impairment of the function of internal organs which may potentially be life threatening.
R have a serious disease of the heart and blood vessels R have low potassium levels
R or someone in your family, has ever had problems with the electrical rhythm of the heart (long QT syndrome)
R have had changes to the rhythm or rate of your heartbeat after taking medicines
Before you receive Primovist, tell your doctor if any of these applies to you. Your doctor will decide whether the intended examination is possible or not.
R Allergy-like reactions may occur after use of Primovist. Severe reactions are possible. Delayed reactions may occur (after hours or days) (see section 4 “Possible Side Effects”).
R Tell your doctor if you have a heart pacemaker or if there are any implants or clips containing iron in your body.
Tell your doctor if:
R your kidneys do not work properly R you have recently had, or soon expect to have, a liver transplant
Your doctor may decide to take a blood test to check how well your kidneys are working before making the decision to use Primovist, especially if you are 65 years of age or older.
Children and adolescents
The safety and efficacy of Primovist have not been established in patients under 18 years old as there is limited experience on its use. Further information regarding the use of Primovist in children is given at the end of the leaflet.
Other medicines and Primovist
Tell your doctor if you are taking, have recently taken or might take any other medicines. These include especially:
R betablockers, medicines used to treat high blood pressure or other heart conditions
R medicines that change the rhythm or rate of your heartbeat (e.g. amiodarone, sotalol)
R rifampicin (medicine used to treat tuberculosis)
Pregnancy and breast-feeding Pregnancy
You must tell your doctor if you think you are or might become pregnant, as Primovist should not be used during pregnancy unless strictly necessary.
Breast-feeding
Tell your doctor if you are breast-feeding or about to start breastfeeding. Your doctor will discuss whether you should continue breast-feeding or interrupt breast-feeding for a period of 24 hours after you receive Primovist.
Primovist contains sodium
Primovist contains 82 mg of sodium per dose (based on the average amount given to a 70 kg person). This should be taken into consideration if you are on a sodium controlled diet.
3. How to use Primovist
Primovist is injected by a doctor via a small needle into a vein. Primovist will be administered immediately before your MRI examination.
After the injection you will be observed for at least 30 minutes. The recommended dose
The actual dosage of Primovist that is right for you will depend on your body weight:
0.1 ml Primovist per kg body weight.
Dosage in special patient groups
The use of Primovist is not recommended in patients with severe kidney problems and patients who have recently had, or soon expect to have, a liver transplant. However if use is required you should only receive one dose of Primovist during a scan and you should not receive a second injection for at least 7 days.
Elderly
It is not necessary to adjust your dose if you are 65 years of age or older but you may have a blood test to check how well your kidneys are working.
Further information regarding the administration and handling of Primovist is given at the end of the leaflet.
If you receive more Primovist than you should
Overdosing is unlikely. If it does happen, the doctor will treat any symptoms that follow.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most of the side effects are mild to moderate.
The most frequently observed side effects in patients receiving Primovist (may affect 5 or more in 1,000 users) are nausea (feeling sick), headache, feeling hot, increased blood pressure, back pain and dizziness.
The most serious side effect in patients receiving Primovist is anaphylactoid shock (a severe allergy-like reaction).
As with other contrast media, in rare cases allergy-like reactions may occur, including severe reactions (shock) in very rare cases, that may need immediate medical intervention.
Mild swelling of the face, lips, tongue or throat, coughing, itching, runny nose, sneezing and hives (nettle-type rash) may be the first signs that a severe reaction is happening. Tell the mRi department staff immediately if you experience any of these signs or have difficulty in breathing.
Delayed reactions hours to days after the administration of Primovist may occur. If this should happen to you, tell your doctor or radiologist.
Below we list the reported/experienced side effects by frequency:
|
Common: may affect up to 1 in 10 people |
Uncommon: may affect up to 1 in 100 people |
Rare: may affect up to 1 in 1,000 people |
Not known: frequency cannot be estimated from the available data |
|
Headache |
Sensation of |
Incapability to |
Fast heartbeat |
|
Nausea |
whirling (vertigo) |
sit or stand |
Restlessness |
|
(feeling |
Dizziness |
still |
Hypersensitivity/ |
|
sick) |
Numbness and |
Tremor |
allergy-like |
|
tingling |
Abnormally |
reaction (e.g. | |
|
Problems with |
strong or rapid |
shock, low | |
|
sense of taste |
heartbeat |
blood pressure, | |
|
Problems with |
(palpitation) |
swelling in the | |
|
sense of smell |
Irregular heartbeat |
tongue or throat, hives | |
|
Flushing |
(signs of heart |
(nettle-type | |
|
Blood pressure |
block) |
rash), swelling | |
|
increased |
Discomfort of |
of the face, | |
|
Breathing |
the mouth |
runny nose, | |
|
difficulties |
Increased |
conjunctivitis, stomach pain, | |
|
Vomiting |
production of |
reduced feeling | |
|
Dry mouth |
saliva |
or sensitivity in | |
|
Skin rash |
Red skin rash |
the skin, | |
|
Severe itching* |
with pimples or spots |
sneezing, cough, itching, pale | |
|
Back pain Chest pain Injection site reactions** Feeling hot Chills Tiredness Feeling abnormal |
Increased sweating Feeling of discomfort Generally feeling unwell |
skin) |
* Severe itching (Generalized itching, Itching of the eye)
** Injection site reactions (various kinds) comprise the following terms: involuntary leakage of the contrast agent and bleeding into the adjacent tissue at the injection site, Injection site burning, Injection site coldness, Injection site irritation, Injection site pain
The following side effects have been life-threatening or fatal in some cases: shock and breathing difficulties.
Changed laboratory values may occur shortly after you have been given Primovist. Therefore inform the health personnel that you have recently undergone an examination with Primovist if you take blood or urine samples.
There have been reports of nephrogenic systemic fibrosis (which causes hardening of the skin and may affect also soft tissue and internal organs) associated with use of other gadolinium-containing contrast agents.
Reporting of side effects
If you get any side effects, talk to your doctor or radiologist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Malta
ADR Reporting
Website: www.medicinesauthority.gov.mt/adrportal
5. How to store Primovist
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the syringe and outer carton label after EXP. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions. This medicine should be used immediately after opening.
This medicine is a clear, colorless to pale yellow solution. It should be visually inspected before use. This medicine should not be used in case of severe discoloration, the occurrence of particulate matter or a defective container.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Primovist contains
R The active substance is gadoxetate disodium. Each ml of solution for injection contains 0.25 mmol gadoxetate disodium (equivalent to 181.43 mg gadoxetate disodium)
R The other ingredients are caloxetate trisodium, trometamol, sodium hydroxide (for pH adjustment) hydrochloric acid (for pH adjustment), and water for injections.
1 prefilled syringe with 5.0 ml contains 907 mg gadoxetate disodium,
1 prefilled syringe with 7.5 ml contains 1361 mg gadoxetate disodium [glass syringe only],
1 prefilled syringe with 10.0 ml contains 1814 mg gadoxetate disodium.
What Primovist looks like and contents of the pack
Primovist is a clear, colourless to pale yellow liquid free from visible particles. The contents of the packs are:
1, 5, or 10 prefilled syringes with 5.0 ml solution for injection (in 10-ml glass prefilled syringes)
1, 5, or 10 prefilled syringes with 7.5 ml solution for injection (in 10-ml glass prefilled syringes) [glass syringe only]
1, 5, or 10 prefilled syringes with 10.0 ml solution for injection (in 10-ml glass prefilled syringes)
Marketing Authorisation Holder
Bayer plc
Bayer House
Strawberry Hill
Newbury
Berkshire
RG14 1JA
Manufacturer
Bayer Pharma AG
Mullerstrasse 178
D - 13353 Berlin, Germany
Telephone: +49 30 468-1111
This medicine is authorised under the name Primovist in the following Member States of the EEA: Austria, Belgium, Croatia, Cyprus, Czech Republic, Estonia, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, United Kingdom.
This leaflet was last revised in November 2015.
The following information is intended for healthcare professionals only:
R Renal impairment
Prior to administration of Primovist, it is recommended that all patients are screened for renal dysfunction by obtaining laboratory tests.
There have been reports of nephrogenic systemic fibrosis (NSF) associated with use of some gadolinium-containing contrast agents in patients with acute or chronic severe renal impairment (GFR< 30ml/min /1.73 m2). Patients undergoing liver transplantation are at particular risk since the incidence of acute renal failure is high in this group. As there is a possibility that NSF may occur with Primovist, it should therefore be avoided in patients with severe renal impairment and in patients in the perioperative liver transplantation period unless the diagnostic information is essential and not available with non-contrast enhanced MRI. If use of Primovist cannot be avoided, the dose should not exceed 0.025 mmol/kg body weight. More than one dose should not be used during a scan. Because of the lack of information on repeated administration, Primovist injections should not be repeated unless the interval between injections is at least 7 days.
As the renal clearance of gadoxetate may be impaired in the elderly, it is particularly important to screen patients aged 65 years and older for renal dysfunction.
Haemodialysis shortly after Primovist administration may be useful at removing Primovist from the body. There is no evidence to support the initiation of haemodialysis for prevention or treatment of NSF in patients not already undergoing haemodialysis.
R Pregnancy and breast-feeding
Primovist should not be used during pregnancy unless the clinical condition of the woman requires use of gadoxetate.
Continuing or discontinuing breast feeding for a period of 24 hours after administration of Primovist, should be at the discretion of the doctor and lactating mother.
R Paediatric population
An observational study was performed in 52 paediatric patients (aged > 2 months and < 18 years). Patients were referred for Primovist enhanced liver MRI to evaluate suspected or known focal liver lesions. Additional diagnostic information was obtained when combined unenhanced and enhanced liver MR images were compared with unenhanced MR images alone. Serious adverse events were reported, however none were assessed by the investigator to be related to Primovist. Due to the retrospective nature and small sample size of this study, no definitive conclusion can be made regarding efficacy and safety in this population.
R Before injection
Primovist is a clear, colourless to pale yellow solution free from visible particles. The contrast medium should be inspected visually before use. Contrast media should not be used in case of severe discolouration, the occurrence of particulate matter or a defective container.
R Administration
Primovist is to be administered undiluted as an intravenous bolus injection at a flow rate of about 2 ml/sec. After the injection the intravenous cannula / line should be flushed using physiological saline solution (9 mg/ml).
P Patient should be observed for at least 30 minutes after the injection.
P Primovist must not be mixed with other medicinal products. P Intramuscular injection must be strictly avoided.
R Handling
Primovist is ready to use.
The prefilled syringe should be prepared for the injection immediately before the examination. The tip cap should be removed from the prefilled syringe immediately before use.
Any solution not used in one examination is to be discarded in accordance with local requirements.
The peel-off tracking label on the syringes should be stuck onto the patient record to enable accurate recording of the gadolinium contrast agent used. The dose used should also be recorded.
If electronic patient records are used, the name of the product, the batch number and the dose should be entered into the patient record.
Glass syringe only:
syringe
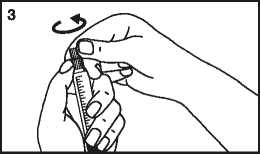
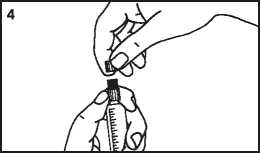
3. Break the protective cover 4. Remove the protective cover
Further information regarding the use of Primovist is given in section 3 of the leaflet.
Packaging Technology Berlin sgmgj_page 1_Bayer Pharma AG
client: 0021 material-no.: 85017187 PZ: 2589C-3 code-no.: 95
name: LF-INS-Primovist 0.25MMOL/ML PFS_country: GB/-/BPH
rotors: Black_
version: 24.11.2015/02 approval: dimension: 297 x 594 mm
0
85017187_02.indd 1
Not all pack sizes may be marketed.
24.11.2015 14:17:28
|
Hda/-/aD :/ujunoD |
Sdd 1W/10WW52‘0 IsiAOUiMd-SNI-dl :aweu |
|
56 rou-apco |
£06852 ^Zd 28X2X058 "OU-|euajew X200 auap |
|
DV euueiid jaAeg |
l abed |buibs uitiag Aboiouipai bu|be>ped |
Primovist® 0.25 mmol/ml Solution for injection, pre-filled syringe

Bayer

Package leaflet: Information for the user
Primovist 0.25 mmol/ml, solution for injection, pre-filled syringe
Gadoxetate, disodium
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
R Keep this leaflet. You may need to read it again.
R If you have any further questions, please ask your doctor giving you Primovist (the radiologist) or the hospital/ MRI-centre personnel.
R If you get any side effects talk to your doctor or radiologist. This includes any possible side effects not listed in this leaflet. See section 4.
Delayed reactions hours to days after the administration of Primovist may occur. If this should happen to you, tell your doctor or radiologist.
Below we list the reported/experienced side effects by frequency:
|
What is in this leaflet | |
|
1. |
What Primovist is and what it is used for |
|
2. |
What you need to know before you are given Primovist |
|
3. |
How to use Primovist |
|
4. |
Possible side effects |
|
5. |
How to store Primovist |
|
6. |
Contents of the pack and other information |
1. What Primovist is and what it is used for
Primovist is a contrast medium for magnetic resonance imaging (MRI) of the liver. It is used to help detect and diagnose changes that may be found in the liver. Abnormal signs within the liver can be better evaluated (as to number, size, and distribution). Primovist can also help the doctor determine the nature of any abnormalities, thereby increasing the confidence one can have in the diagnosis.
It is provided as a solution for intravenous injection. This medicine is for diagnostic use only.
MRI is a form of medical diagnostic imaging that forms pictures after water molecules have been detected in normal and abnormal tissues. This is done using a complex system of magnets and radiowaves.
2. What you need to know before you are given Primovist Do not use Primovist
R if you are allergic to gadoxetate disodium or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you are given Primovist if you R have or have had allergy (e.g. hay fever, hives) or asthma R had a previous reaction to contrast media R have a poor kidney function.
The use of some gadolinium-containing contrast agents in patients with these conditions has been associated with a disease called Nephrogenic Systemic Fibrosis (NSF). NSF is a disease involving thickening of the skin and connective tissues. NSF may result in debilitating joint immobility, muscle weakness or impairment of the function of internal organs which may potentially be life threatening.
R have a serious disease of the heart and blood vessels R have low potassium levels
R or someone in your family, has ever had problems with the electrical rhythm of the heart (long QT syndrome)
R have had changes to the rhythm or rate of your heartbeat after taking medicines
Before you receive Primovist, tell your doctor if any of these applies to you. Your doctor will decide whether the intended examination is possible or not.
R Allergy-like reactions may occur after use of Primovist. Severe reactions are possible. Delayed reactions may occur (after hours or days) (see section 4 “Possible Side Effects”).
R Tell your doctor if you have a heart pacemaker or if there are any implants or clips containing iron in your body.
Tell your doctor if:
R your kidneys do not work properly R you have recently had, or soon expect to have, a liver transplant
Your doctor may decide to take a blood test to check how well your kidneys are working before making the decision to use Primovist, especially if you are 65 years of age or older.
Children and adolescents
The safety and efficacy of Primovist have not been established in patients under 18 years old as there is limited experience on its use. Further information regarding the use of Primovist in children is given at the end of the leaflet.
Other medicines and Primovist
Tell your doctor if you are taking, have recently taken or might take any other medicines. These include especially:
R betablockers, medicines used to treat high blood pressure or other heart conditions
R medicines that change the rhythm or rate of your heartbeat (e.g. amiodarone, sotalol)
R rifampicin (medicine used to treat tuberculosis)
Pregnancy and breast-feeding Pregnancy
You must tell your doctor if you think you are or might become pregnant, as Primovist should not be used during pregnancy unless strictly necessary.
Breast-feeding
Tell your doctor if you are breast-feeding or about to start breastfeeding. Your doctor will discuss whether you should continue breast-feeding or interrupt breast-feeding for a period of 24 hours after you receive Primovist.
Primovist contains sodium
Primovist contains 82 mg of sodium per dose (based on the average amount given to a 70 kg person). This should be taken into consideration if you are on a sodium controlled diet.
3. How to use Primovist
Primovist is injected by a doctor via a small needle into a vein. Primovist will be administered immediately before your MRI examination.
After the injection you will be observed for at least 30 minutes. The recommended dose
The actual dosage of Primovist that is right for you will depend on your body weight:
0.1 ml Primovist per kg body weight.
Dosage in special patient groups
The use of Primovist is not recommended in patients with severe kidney problems and patients who have recently had, or soon expect to have, a liver transplant. However if use is required you should only receive one dose of Primovist during a scan and you should not receive a second injection for at least 7 days.
Elderly
It is not necessary to adjust your dose if you are 65 years of age or older but you may have a blood test to check how well your kidneys are working.
Further information regarding the administration and handling of Primovist is given at the end of the leaflet.
If you receive more Primovist than you should
Overdosing is unlikely. If it does happen, the doctor will treat any symptoms that follow.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most of the side effects are mild to moderate.
The most frequently observed side effects in patients receiving Primovist (may affect 5 or more in 1,000 users) are nausea (feeling sick), headache, feeling hot, increased blood pressure, back pain and dizziness.
The most serious side effect in patients receiving Primovist is anaphylactoid shock (a severe allergy-like reaction).
As with other contrast media, in rare cases allergy-like reactions may occur, including severe reactions (shock) in very rare cases, that may need immediate medical intervention.
Mild swelling of the face, lips, tongue or throat, coughing, itching, runny nose, sneezing and hives (nettle-type rash) may be the first signs that a severe reaction is happening. Tell the mRi department staff immediately if you experience any of these signs or have difficulty in breathing.
|
Common: may affect up to 1 in 10 people |
Uncommon: may affect up to 1 in 100 people |
Rare: may affect up to 1 in 1,000 people |
Not known: frequency cannot be estimated from the available data |
|
Headache |
Sensation of |
Incapability to |
Fast heartbeat |
|
Nausea |
whirling (vertigo) |
sit or stand |
Restlessness |
|
(feeling |
Dizziness |
still |
Hypersensitivity/ |
|
sick) |
Numbness and |
Tremor |
allergy-like |
|
tingling |
Abnormally |
reaction (e.g. | |
|
Problems with |
strong or rapid |
shock, low | |
|
sense of taste |
heartbeat |
blood pressure, | |
|
Problems with |
(palpitation) |
swelling in the | |
|
sense of smell |
Irregular heartbeat |
tongue or throat, hives | |
|
Flushing |
(signs of heart |
(nettle-type | |
|
Blood pressure |
block) |
rash), swelling | |
|
increased |
Discomfort of |
of the face, | |
|
Breathing |
the mouth |
runny nose, | |
|
difficulties |
Increased |
conjunctivitis, stomach pain, | |
|
Vomiting |
production of |
reduced feeling | |
|
Dry mouth |
saliva |
or sensitivity in | |
|
Skin rash |
Red skin rash |
the skin, | |
|
Severe itching* |
with pimples or spots |
sneezing, cough, itching, pale | |
|
Back pain Chest pain Injection site reactions** Feeling hot Chills Tiredness Feeling abnormal |
Increased sweating Feeling of discomfort Generally feeling unwell |
skin) |
* Severe itching (Generalized itching, Itching of the eye)
** Injection site reactions (various kinds) comprise the following terms: involuntary leakage of the contrast agent and bleeding into the adjacent tissue at the injection site, Injection site burning, Injection site coldness, Injection site irritation, Injection site pain
The following side effects have been life-threatening or fatal in some cases: shock and breathing difficulties.
Changed laboratory values may occur shortly after you have been given Primovist. Therefore inform the health personnel that you have recently undergone an examination with Primovist if you take blood or urine samples.
There have been reports of nephrogenic systemic fibrosis (which causes hardening of the skin and may affect also soft tissue and internal organs) associated with use of other gadolinium-containing contrast agents.
Reporting of side effects
If you get any side effects, talk to your doctor or radiologist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Malta
ADR Reporting
Website: www.medicinesauthority.gov.mt/adrportal
5. How to store Primovist
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the syringe and outer carton label after EXP. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions. This medicine should be used immediately after opening.
This medicine is a clear, colorless to pale yellow solution. It should be visually inspected before use. This medicine should not be used in case of severe discoloration, the occurrence of particulate matter or a defective container.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Primovist contains
R The active substance is gadoxetate disodium. Each ml of solution for injection contains 0.25 mmol gadoxetate disodium (equivalent to 181.43 mg gadoxetate disodium)
R The other ingredients are caloxetate trisodium, trometamol, sodium hydroxide (for pH adjustment) hydrochloric acid (for pH adjustment), and water for injections.
1 prefilled syringe with 5.0 ml contains 907 mg gadoxetate disodium,
1 prefilled syringe with 10.0 ml contains 1814 mg gadoxetate disodium.
What Primovist looks like and contents of the pack
Primovist is a clear, colourless to pale yellow liquid free from visible particles. The contents of the packs are:
1, 5, or 10 prefilled syringes with 5.0 ml solution for injection (in 10-ml plastic prefilled syringes)
1, 5, or 10 prefilled syringes with 10.0 ml solution for injection (in 10-ml plastic prefilled syringes)
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Bayer plc
Bayer House
Strawberry Hill
Newbury
Berkshire
RG14 1JA
Manufacturer
Bayer Pharma AG Mullerstrasse 178 D - 13353 Berlin, Germany Telephone: +49 30 468-1111
This medicine is authorised under the name Primovist in the following Member States of the EEA: Austria, Belgium, Croatia, Cyprus, Czech Republic, Estonia, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, United Kingdom.
This leaflet was last revised in November 2015.
As the renal clearance of gadoxetate may be impaired in the elderly, it is particularly important to screen patients aged 65 years and older for renal dysfunction.
Haemodialysis shortly after Primovist administration may be useful at removing Primovist from the body. There is no evidence to support the initiation of haemodialysis for prevention or treatment of NSF in patients not already undergoing haemodialysis.
R Pregnancy and breast-feeding
Primovist should not be used during pregnancy unless the clinical condition of the woman requires use of gadoxetate.
Continuing or discontinuing breast feeding for a period of 24 hours after administration of Primovist, should be at the discretion of the doctor and lactating mother.
R Paediatric population
An observational study was performed in 52 paediatric patients (aged > 2 months and < 18 years). Patients were referred for Primovist enhanced liver MRI to evaluate suspected or known focal liver lesions. Additional diagnostic information was obtained when combined unenhanced and enhanced liver MR images were compared with unenhanced MR images alone. Serious adverse events were reported, however none were assessed by the investigator to be related to Primovist. Due to the retrospective nature and small sample size of this study, no definitive conclusion can be made regarding efficacy and safety in this population.
R Before injection
Primovist is a clear, colourless to pale yellow solution free from visible particles. The contrast medium should be inspected visually before use. Contrast media should not be used in case of severe discolouration, the occurrence of particulate matter or a defective container.
R Administration
Primovist is to be administered undiluted as an intravenous bolus injection at a flow rate of about 2 ml/sec. After the injection the intravenous cannula / line should be flushed using physiological saline solution (9 mg/ml).
P Patient should be observed for at least 30 minutes after the injection.
P Primovist must not be mixed with other medicinal products. P Intramuscular injection must be strictly avoided.
R Handling
Primovist is ready to use.
The prefilled syringe should be prepared for the injection immediately before the examination. The tip cap should be removed from the prefilled syringe immediately before use.
Any solution not used in one examination is to be discarded in accordance with local requirements.
The peel-off tracking label on the syringes should be stuck onto the patient record to enable accurate recording of the gadolinium contrast agent used. The dose used should also be recorded.
If electronic patient records are used, the name of the product, the batch number and the dose should be entered into the patient record.
Plastic syringe only:
Hand injection
Injection with a power injector
Packaging Technology Berlin sgmgj_page 1_Bayer Pharma AG
client: 0021 material-no.: 84863521 PZ: 2589C-3 code-no.: 313
name: LF-INS-Primovist 0.25MMOL/ML PFSP_country: GB/-/BPH
rotors: Black_
version: 19.11.2015/04 approval: dimension: 297 x 594 mm
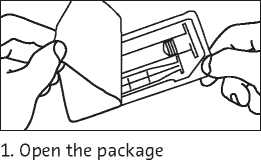
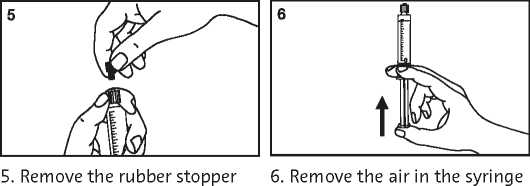
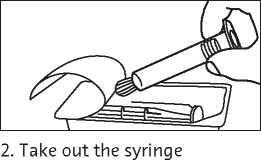
2. Take out the syringe and plunger rod
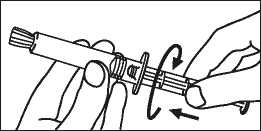
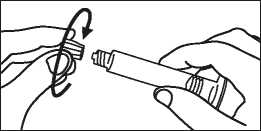
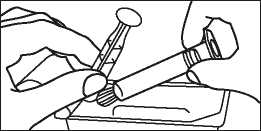
3. Turn the plunger rod clockwise into the syringe
3. Open the cap with a twist
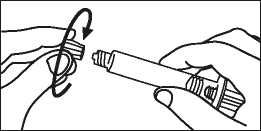

4. Open the cap with a twist
4. Connect the tip of the syringe to the tubing system by turning it clock-wise. Proceed according to the instructions for the device
0
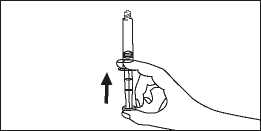
5. Remove the air in the syringe
Further information regarding the use of Primovist is given in section 3 of the leaflet.
The following information is intended for healthcare professionals only:
R Renal impairment
Prior to administration of Primovist, it is recommended that all patients are screened for renal dysfunction by obtaining laboratory tests.
There have been reports of nephrogenic systemic fibrosis (NSF) associated with use of some gadolinium-containing contrast agents in patients with acute or chronic severe renal impairment (GFR< 30ml/min /1.73 m2). Patients undergoing liver transplantation are at particular risk since the incidence of acute renal failure is high in this group. As there is a possibility that NSF may occur with Primovist, it should therefore be avoided in patients with severe renal impairment and in patients in the perioperative liver transplantation period unless the diagnostic information is essential and not available with non-contrast enhanced MRI. If use of Primovist cannot be avoided, the dose should not exceed 0.025 mmol/kg body weight. More than one dose should not be used during a scan. Because of the lack of information on repeated administration, Primovist injections should not be repeated unless the interval between injections is at least 7 days.
84863521_04.indd 1
19.11.2015 08:25:30
|
Hda/-/aD :/ujunoD |
dSdd IW/IOWWSZ‘0 )S|AOUi|Jd-SNI-dl :aweu |
|
£X£ "ou-apco |
£06852 ^Zd X2S£981?8 "ou-ieuajew X200 auap |
|
DV euueiid jaAeg |
l abed |buibs uitiag Aboiouipai bm be jpeg |
Primovist® 0.25 mmol/ml Solution for injection, pre-filled syringe

Bayer