Prismasol 2 Mmol/L Potassium Solution For Haemodialysis/Haemofiltration
Prismasol® 2
UK, IE, MT Package leaflet: Information for the user,............................3
HU Betegtajekoztato; Informaciok a felhasznalo szamara........5
PL Ulotka dla pacjenta..............................................................7
UK, IE, MT The following information is intended
for healthcare professionals only.........................................9
HU Az alabbi informaciok kizarolag
egeszsegugyi szakembereknek szolnak...........................11
PL Informacje przeznaczone wyfgcznie
dla fachowego personelu medycznego............................13
'° O -Ok - C'K
DC57S30C1 Rev. 2013-03 4746 GAMBRO
Package leaflet: Information for the user
PrismasoP 2 mmol/l Potassium Solution for haemodialysis/ haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
» if you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
WHAT IS IN THIS LEAFLET:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. WHAT PRISMASOL IS AND WHAT IT IS USED FOR
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution S0% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltra-tion (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances. Prismasol 2 mmol/l Potassium is indicated particularly in patients who have tendency to hyperkalaemia (a high concentration of potassium in the blood).
2. WHAT YOU NEED TO KNOW BEFORE YOU ARE GIVEN PRISMASOL 1 2
OTHER MEDICINES AND PRISMASOL
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hyper-calcaemta (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
PREGNANCY AND BREASTFEEDING
If you are pregnant or breastfeeding, trunk you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
DRIVING AND USING MACHINES
Prismasol is not known to affect your ability to drive or use machines.
3, HOW PRISMASOL IS USED
Always use this medicine exactly as your doctor or pharmacist has told you, Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend or: your clinical condition
|
mmol/l |
mEq/l | |
|
Calcium, Ca:" |
~ 175 |
3.50 |
|
Magnesium. Mg2" |
0.50 |
1.00 |
|
Sodium. Na2 |
140.00 |
140.00 |
|
Chloride, Cl~ |
111.50 |
111.50 |
|
Lactate |
3.00 |
3 00 |
|
Hydrogen carbonate, HCOy |
32.00 |
32.00 |
|
Potassium, K2 |
2.00 |
2.00 |
|
Glucose |
6.10 |
and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor. Administration route: Intravenous use and for haemodialysis,
For instructions for use, please see section 'The following information is intended for healthcare professionals only’’.
IF YOU THINK YOU ARE GIVEN MORE PRISMASOL THAN YOU THINK YOU SHOULD BE
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure. Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes, in case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hy-pophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration). Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. HOW TO STORE
PRISMASOL_
Keep this medicine out of the sight and reach of children.
Do not store below +4=C.
Do not use this medicine after the expiry dale which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
WHAT PRISMASOL CONTAINS The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A))
contains
Calcium chloride dihydrate 5.145 g Magnesium chloride hexahydrate 2.033 g
1000 ml of buffer solution (from the large compartment (B)) contains Sodium chloride 6.450 g
Sodium hydrogen
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
Theoretical Osrnolarity:
297 mOsm/l
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution:
7.0-8.5
WHAT PRISMASOL LOOKS LIKE AND CONTENTS OF THE PACK
Prismasol is presented in a two-compartment bag containing in the smaller compartment A. the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A-'-B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
MARKETING AUTHORISATION HOLDER:
Gambro Lundia AB Magistratsvagen 16 SE-220 10 Lund SWEDEN
MANUFACTURER:
Gambro Dasco S.p.A.
Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012
Betegtajekoztato: Informaciok a felhasznalo szamara
Prismasol4' 2 mmol/l k&lium Hemodializaio vagy hemofiltracios oldat
Kaldum-klorid-dihidrat/ Magnezium-klorid-hexahidrat/ Glukdz-monohidrat/ 90% terfogat-szazalekos tejsavoldat/ Natrium-klorid/ Kalium-klorid/ Natrium-hidrogen-karbonat Mielott beadnak Ortnek ezt a gyogyszert, olvassa ei figyelmesen az alabbi betegtajekoztatdt, mely az On szamara fontos inform eiciokat tartalmaz.
• Tartsa meg a betegtajekoz-tatot, mert a benne szereplo informaciokra a kesobbiekben is szuksege lehet.
• Tovabbi kerdeseivel forduljon ke-zelborvosahoz, gyogyszeresze-hez vagy a szakszemelyzethez.
• Ha Onnel barmilyen mellekha-tas jelentkezik. tajekoztassa errdl kezeloorvosat, gydgysze-reszet vagy a szakszernelyze-tet. Ez a betegtajekoztatoban fel nern sorolt barmilyen lehetseges mellekhatasra is vonatkozik.
A BETEGTAJEKOZTATO TARTALMA:
1. Milyen tipusu gyogyszer a Prismasol es milyen betegsegek eseten aikalmazhato?
2. Tudnivalok a Prismasol alkalmazasa elbtt
3. Hogyan kell aikalmazni a Prismasolt?
4. Lehetseges mellekhatasok
5. Hogyan kell a Prismasolt tarolni?
6. A csomagolas tartalrna es egyeb informaciok
1. MILYEN TIPUSU
GYOGYSZER A PRISMASOL ES MILYEN BETEGSEGEK ESETEN ALKALMAZHATO?
A Prismasol a kdvetkezo hato-anyagokat tartalmazza: kalcium-klorid-dihidrat, magnezium-klorid-hexahidrat, glukoz-monohidrat.
90% terfogat-szazalekos tejsavoldat, natrium-klorid, kalium-klorid es natrium-hidrogen-karbonat,
A Prismasol a veseelegte-lensegek kezeleseben alkal-mazott hemofiltracios vagy hemodiafiltracios oldat (a szuron at-halado verba! fellepo folyadekvesz-teseg potlasara), illetve folyamatos hemodializis vagy hemodiafiltraeio soran alkalmazott oldat (amikor is a ver a dializalo membran egyik oldalan aramiik, a hemodializaio oldat pedig a masikon).
A Prismasol oldat aikalmazhato dializalhato vagy sziirhetd anya-goktol elszenvedett gydgyszermer-gezes kezeleseben is.
A Prismasol 2 mmol/l kalium alkalmazasa kuldndsen hiperkalemiara hajlamos betegeknel javallott (olyan betegeknel, akiknel fennall annak kockazata, hogy a ver tulzottan magas kaliumszintje alakuljon ki).
2, TUDNIVALOK A PRISMASOL ALKALMAZASA ELOTT 3 4
hemodializissel tortend kezeleseben jartas orvos altal szeme-lyesen - vagy az 6 felugyeletevel - aikalmazhato.
A kezeles elott es alatt ellenorzik a ver parametereit, pi. a sav-b^zis haztartast es a verben az elektroli-tokat (a sok koncentraciojat),
A vercukorszintet folyamatosan monitorozni kell, kuldndsen cukor-betegek eseteben.
EGYEB GYOGYSZEREK ES A PRISMASOL
Feltetlenul tajekoztassa kezeloorvosat vagy gyogyszereszet a jelen-leg vagy nemreglben alkalmazott. valamint aikalmazni tervezett egyeb gyogyszereirol.
Ez azdrt fontos, mert egyes gybgy-szerek koncentracioja csdkkenhet a verben a kezeles hatasara. Kezeloorvosa eldonti majd. hogy szukseges-e gyogyszeres kezele-senek barmilyen modositasa. Kiildnosen arrol fontos tajekoztat-nia kezeloonfosat, ha a kdvetkezo gyogyszerek valamelyiket szedi:
• Digitalisz gyogyszerek (kuion-bdzd sziv-rendellenessegek kezelesere), mivel a digitalisz gyogyszerek altal keltett aritmia (szabalytalan ritmusu vagy till gyors szivveres) kockazata megno hipokalemias allapo-toknal (amikor alacsony a ver kallumkoncentraclbja).
• D-vitamin es egyeb, kalciumot tartalmazo gyogyaszati keszit-menyek; mivel ezek novelhetik a ver magas kalciumkoncent-raciojanak (hlperkalcemia) kockazatat.
• Egyeb olyan gyogyszerek, amelyek natrium-hidrogen-karbonatot is tartalmaznak, mert ezek alkalmazasa eseten megno a metabolikus alkalozis (tulzott bikarbonatmennyiseg a verben) kockazata.
TERHESSEG ES SZOPTATAS
Ha On terhes vagy szoptat, illetve ha fennall Onnel a terhesseg lehe-tosege vagy gyermeket szeretne, a gyogyszer alkalmazasa elott beszeljen kezeloorvosaval vagy gyogyszereszevel.
Amennyiben On terhes vagy szoptat, minden egyeb gyogyszerhez hasonloan kezeloorvosa donti el, hogy kaphat-e Prismasolt vagy sem.
A KESZiTMENY HATASAI A GEPJARMUVEZETESHEZ ES GEPEK KEZELESEHEZ SZUKSEGES KEPESSEGEKRE
A, Prismasol eseteben nem allapf-tottak meg, hogy hatassa! lenne a gepjarmuvezeteshez es gepek keze-lesehez szukseges kepessegekre.
3. HOGYAN KELL ALKALMAZNI A PRISMASOLT?_
A gyogyszert mindig a kezeloorvosa vagy gyogyszeresze altal elmon-dottaknak megfeleloen alkalmazza. Amennyiben nem biztos az adago-last illetoen, kerdezze meg kezelo-orvosat vagy gyogyszereszet.
A Prismasol alkalmazando meny-nyisege a beteg klinikai allapatatcl es az elerni kivant folyadekegyen-sulytol fugg. Az alkalmazando mennyiseget ezert a kezeloorvos megitelese hatarozza meg.
Az alkalmazas modja: Intravenas alkalmazasra es hemodializishez,
A hasznalati utmutatot last! ,.Az alabbi informaciok kizarolag egesz-segugyi szakembereknek szolnak" cirnii reszben,
HAAZ ELOIRTNAL TQBB PRISMASOLT KAPOTT
Az eljaras ideje alatt gondosan monitorozzak a folyadekegyensulyt. valamint az elektrolit- es a sav-ba-zis egyensulyt.
A tuladagolas folyadekterheleshez vezethet. ha veseelegtelenseaben szenved.
A tuladagolas sulyos kbvetkezme-nyeket okozhat. mint a parigasos szivelegtelenseg, illetve az elektro-lit- es sav-bazis zavarok.
A hemofiltracio folyamatos alkal-mazasa megszunteti a foiyadek-es elektrolitfelesleget. Folyadek tultoltes eseten az ultrafiltraciot noveln kell, tovabba csokkenteni kell a hemofiltraclds oldat adagolasanak se-besseget, Sulyos kiszaradas eseten az ultrafiltraciot el kell hagyni. illetve megfelelo mertekben nbvelni kell a hemofiltracios oldat bearamlasat Ha barmilyen tovabbi kerdese van a gyogyszer alkalmazasaval kapcsoiatban, kerdezze meg keze-loo^osat, gyogyszereszet vagy a szakszemelyzetet.
4. LEHETSEGES IVIELLEKHATASOK
Mint minden gyogyszer. igy ez a gyogyszer is okozhat mellekha-tasokat. amelyek azonban nem mindenkinel jelentkeznek,
Az alabbi nemklvanatos hatasok azonban elkepzelhetdk lehetnek az oldat aikalmazasakor: A viz korosan magas vagy alacsony mennyisege a testben (hiper- vagy hipohidracio), a verben oldott sokzavarai (elektrolitzavarok), a foszfatok rendellenesen alacsony koncentracioja a verben (hipofoszfatemia), korosan magas vercukorszint (hiperglikemia), a ver vegyhatasanak lugos iranyu eltolodasa (metabolikus alkalozis. a piazma bikarbonat-koncentracioja-nak megemelketieseert elsodlege-sen felelos folyamat).
A diallziskezelesekkel kapcsoiatban fellephet nehany nemklvanatos hatas. mint peldaul hanyinger (emelyges), hanyas (rosszullet), izomgorcsok es alacsony vernyo-mas (hipotenzio).
Ha Onnel barmilyen mellekhatas jelentkeziK. tajekoztassa kezeloorvo-sat, gyogyszereszet vagy a szakszemelyzetet. Ez a betegtdjekoztatoban tel nem sorolt bdrmilyen lehetseges meliekhatasra is vonatkozik.
5. HOGYAN KELL A
PRISMASOLT TAROLNI?
A gyogyszer gyermekekiol elzarva tartando!
Ne tarolja +4°C alatti homersek-leten.
A cimken es a csomagolason feltuntetett lejarati ido utan ne alkalmazza ezt a gyogyszert. A lejarati ido a megadatt honap utolso napjara vonatkozik.
6. A CSOMAGOLAS TARTALMA ES EGYEB INFORMACIOK_
MIT TARTALMAZ A PRISMASOL A keszitmeny hatoanyagai: Osszekeveres elatt:
A kisebbik (A) rekeszben levo elektrolitoldat tartalma 1000 ml-re vetltve:
Kalcium-klorid-dihidrat 5.145 g
Magnezium-kiorid-hexahidrat 2,033 g
A nagyobbik (B) rekeszben levo pufferoldat tartalma 1000 ml-re vetitve:
Natrium-hidrogen-karbonat 3,090 g
Osszekeveres utan:
Az A rekeszben levo oldat (250 ml) es a B rekeszben levo oldat (4750 ml) dsszekeverese utan a vegleges oldat (5000 ml) tartalma:
|
mmol/l |
mEq/l | |
|
Kalcium, Ca:4 |
1,75 |
3,50 |
|
Magnezium, Mg:’ |
O.oC |
1.00 |
|
Natrium, Na" |
140,00 |
140,00 |
|
Klorid, Cl" |
111,50 |
111,50 |
|
Laktat |
3.00 |
3.00 |
|
Hidrogen-karbonat, HCOu |
32,00 |
32.00 |
|
Kalium. K' |
2,00 |
2,00 |
|
Glukoz |
6.10 |
Elmeleti ozmolaritas: 297 mOsm/l Egyeb bsszetevok: szen-dioxid, illetve injekciohoz valo viz Az osszekevert oldat kemhatasa (pH): 7 0-8 5
MILYEN A PRISMASOL KESZITMENY KULLEME ES MIT TARTALMAZ A CSOMAGOLAS
A Prismasol ketrekeszes zsak formatumu kiszereiesben kerul forgalomba: a kisebbik (A) rekesz tartalmazza az eiektrolitoldatot. a nagyobbik (B) rekesz pedig a pufferoldatot. Az alkalmazasra elokeszitett, vegleges oldat a felnyithato lezaras szettorese (rtyi-tasa) utan a ket oldat osszekevere-desevel jon letre. Az alkalmazasra kesz oldat tiszta es enyhen sargas szinu. A ket zsak (A+B) 5000 ml oldatot tartalmaz hemofiltraciohoz es hemodializishez. A zsak atlatszo foliaval is be van csomagolva. Mindegyik doboz ket zsakot es egy betegtajekoztatot tartalmaz.
A FORGALOMBA HOZATALI ENGEDELY JOGOSULTJA:
Gambro Lundia AB Magistratsvagen 16 SE-220 10 Lund SVEDORSZAG
GYARTO:
Gambro Dasco S.p.A.
Sondalo Plant Via Stelvio 94 23035 Sondalo (SO) OLASZORSZAG
OGYI-T-21178/02 2 x 5000 ml tobbretegu poliolefin anyagbol keszult ketrekeszes (A es B) zsakba tdltve, csatlakozo-csappal.
A betegtajekoztato legutobbi feiulvizsgalatanak datuma:
2012 oktober
Ulotka dla pacjenta
Prismasol 2 mmol/l potasu Roztwor do hemodializy/ hemofiltracji
Wapnia chlorek dwuwodny/ magne-zu chlorek szesciowodny/ glukoza jednowodna/ kwasu mlekowego roztwor 90% w/w / sodu chlorek/ potasu chlorek/ sodu wodorowe-glan
Nalezy zapoznac sie z tresci^ ulotki przed przyjeciem leku, poniewaz zawiera ona informacje wazne dla pacjenta.
• Nalezy zachowac t§ ulotke, aby w razie potrzeby mod ja ponow-nie przeczytac.
• Nalezy zwrocic sie do lekarza farmaceuty lub pielegniarki w razie jakichkclwiek dalszych watpliwosci.
• Jesli nasill sig ktorykolwiek z objawow niepozadanych lub wystqpiq jakiekolvviek objawy niepoz^dane niewymienione w ulotce, nalezy powiadomic lekarza lub farmaceute.
SPIS TRESCI ULOTKI:
1. Co to jest Prismasol 2 mmol/l potasu I w jakim celu sig go stosuje
2. Informacje wazne przed przyjeciem roztworu Prismasol 2 mmol/l potasu
3. Jak stosowac Prismasol 2 mmol/l potasu
4. Mozliwe dzialania niepozadane
5. Jak przechowywac Prismasol 2 mmol/l potasu
6. Zawartosc opakowania i inne informacje
1. CO TO JEST
PRISMASOL 2 MMOL/L POTASU I W JAKIM CELU SIE; GO STOSUJE_
Prismasol zawiera substancje czynne: wapnia chlorek dwuwodny, magnezu chlorek szesciowodny, glukoza jednowodna, kwasu mlekowego roztwor 90% w/w, sociu chlorek, potasu chlorek, sodu wodorowegian.
Prismasol 2 mmol/l potasu jest sto-sowany w leczeniu niewydolnosci nerekjako roztwor do hemofiltracji lub hemodiafiltracji (jako roztwor zast^pczy przy utracie ptynow z krwi przeplywajdcej przez filtr) oraz ci^glej hemodializy lub hemodiafiltracji (krew przeptywa po jednej stionie membrany dializacyjnej. podczas gdy roztwor do hemodializy przeptywa po drugiej stronie membrany).
Prismasol 2 mmol/l potasu mozna rowniez stosowac w przypadku zatrucia lekami zawieraj^cymi substancje ulegaja.ee dializie lub filtracji.
Prismasol 2 mmol/l potasu jest wskazany szczegolnie u pacjentow. majgcych sktonnosc do hiperkalie-mii (wysokie stezenie potasu we
krwi).
2. INFORMACJE WAZNE PRZED PRZYJECIEM ROZTWORU PRISMASOL 2 MMOL/L POTASU 5 6
Roztwor powinlen bye uzywany wyf^eznie przez lub pod nadzo-rem lekarza wykvvalifikowanego w leczeniu niewydolnosci nerek przez zastosowanie hemofiltracji, hemodiafiltracji i ciggtej hemodializy, Przed zastosowaniem leczenia i podczas niego zostanie zbadana krew pacjenta, np. rownowaga kwasowo-zasadowa i stezenie elektrolitow (soli we krwi).
Nalezy doktadnie monltorowac stg-zenie glukozy we krwi, zwlaszcza gdy paejent choruje na oukrzyeg.
INNE LEKII
PRISMASOL 2 MMOL/L POTASU
Nalezy powiedziec iekarzowi lub farmaceucie o wszystkich lekach przyjmowanych obecnie lub ostat-nio a takze o lekach, ktore paejent planuje przyjmowac.
Stezenie we krwi innych przyjmowanych lekow moze zostac obnizone podczas leczenia. Lekarz zdecyduje, czy wymagana jest zmiana przyjmowanych lekow.
W szczegoinosci nalezy powiedziec Iekarzowi o przyjmowaniu nastepu-jacych lekow:
• glikozydow (stosowanych w leczeniu okreslonych chorob serca). gdyz podczas hipokalie-mii (obnizonego stezenia potasu we krwi) zwigkszaja one ryzyko wystapienia zaburzeri rytmu serca (nleregularnego lub przy-spieszonego bicia serca);
• witaminy D i produktow lecz-niezyeh zawierajacych vvaph, gdyz moga one zwi$kszac ryzyko wystapienia hiperkalce-mii (wysokiego stezenia wapnia we krwi);
• jakichkolwiek dodatkow wodoroweglanu sodu obecnych w innych lekach, gdyz moze on zwifjkszac ryzyko wystapienia zasadowicy metabolieznej (nadmiaru wodoroweglanow we krwi),
CIAZA I KARMIENIE PIERSIA
W ciazy i w okresie karmienia pier-sia lub gdy istnieje podejrzenie, ze kobieta jest w ciazy, lub gdy planuje ciaz?, przed zastosowaniem tego leku nalezy poradzic sie lekarza lub farmaceuty.
Tak jak w przypadku wszystkich lekow, lekarz zadecyduje o poda-
6. ZAWARTOSC OPAKOWANIA I INNE INFORMACJE___
CO ZAWIERA
PRISMASOL 2 MMOL/L POTASU Substancje czynne:
Przed odtworzeniem/zwiesza-niem:
1000 ml roztworu elektrolitowego
mmol/l mEq/l 1,75 3,50
0,50 100
140,00 140,00 111,50 111,50
3.00 3,00 w 32,00 32,00
2.00 2 00 6,10
waniu roztworu Prismasol kobietom w ciazy lub karmiqcym piersiq.
PROWADZENiE POJAZDOW I OBStUGA MASZYN
Nie wiadomo, czy Prismasol wplywa na zdolnosc prowadzenia pojazdow i obslugi maszyn.
3. JAK STOSOWAC PRISMASOL 2 MMOL/L POTASU_
Ten lek nalezy zawsze stosowac zgodnie z zaleceniami lekarza. W razie watpiiwosci nalezy zwrocic sip do lekarza lub farmaceuty.
Objptosc uzywanego roztworu Prismasol 2 mmol/l potasu zalezy od stanu klinicznego pacjenta i docelowego bilansu plynow, Dlatego decyzjg o objgtosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Droga podawania: do podawania dozylnego i hemodializy.
Instrukcje uzycia znajduja. sie w cz^sci Jnformacje przeznaczone wylacznie dla faohowego personelu medycznego".
GDY PACJENTOWI WYDAJ^
SIE, ZE PODANO MU WIEKSZA ILOSC ROZTWORU PRISMASOL NIZ ZALECANA
Nalezy doktadnie monitorowac bilans plynow, rownowage eiektroli-towa i kwasowo-zasadowq. Przedawkowanie moze spowodo-wac przeciqzenie ptynami u pacjen-tow z niewydalnosciq nerek. przedawkowanie moze prowadzic do powaznych nastepstw, takich jak zastoinowa niewydolnosc serca, zaburzenia rownowagi elektrolito-wej czy kwasowo-zasadowej.
Giggle stosowanie hemofiltracji usuwa nadmiar plynu i elektrolitow. W przypadku przewodnienia nalezy zwiekszyc uitrafiltracjq i zmniejszyc wielkosc przeplywu roztworu do hemofiltracji. W przypadku ciez-kiego odwodnienia konieczne jest przerwanie ultrafiltracji i odpowied-nie zwiekszenie naptywu roztworu do hemofiltracji.
W razie jakichkotwiek dalszych watpiiwosci zwiqzanych ze stoso-waniem tego leku nalezy zwrocic sie do lekarza, farmaceuty lub pielegniarki.
4. MOZLIWE DZIAtANIA
NIEPOZADANE_
Jak kazdy lek, lek ten moze powo-dowac dziatania niepozadane, cho-ciaz nie u kazdego one wysta.pia. Mozliwe sq nastppujace dzialania niepozadane zwiqzane z uzywa-niem roztworu: przewodnienie lub odwodnienie (nieprawidtowo vvysoka lub niska objgtosc wody w organizmie), zaburzenia elektro-litowe (sole mineralne we krwi), hipofosfatemia (nieprawidtowo niskie stqzenie fosforanow we krwi), hiperglikemia (nieprawidtowo wysokie stezenie glukozy we krwi) i alkaloza metaboliczna (proces, ktory powoduje glownie zwigksze-nie stezenia wodorow^glanu w osoczu),
Moga wystqpic pewne reakcje niepozqdane zwiqzane z dializa, np, nudnosci, vvymioty. skurcze miesni oraz niedocisnienie (niskie cisnienie krwi).
Jesli wystqpig jakiekolwiek objawy niepozadane, w tym wszelkie mozliwe objawy niepozgdana niewymienione w ulotce. nalezy zwrocic sip do lekarza. farmaceuty lub pielegniarki.
5. JAK PRZECHOWYWAC PRISMASOL 2 MMOL/L POTASU_
Lek nalezy przechowywab w miej-scu niewidocznym i niedostepnym dla dzieci.
Nie przechowywac w temperaturza ponizej +4'C.
Nie stosowac tego leku po uplywie terminu waznosci zamieszczonego na etykiecie i opakowaniu. Termin
waznosci oznacza ostatni dzion
danego miesiaca.
(mala komora A) zawiera:
Wapnia chlorek dwuwodny 5.145 g Magnezu chlorek
1000 ml roztworu buforovvego (duza komora B) zawiera:
Sodu chlorek 6 450 g
Sodu wodoroweglan 3 090 g
Potasu chlorek 0,157 g
Po odtworzeniu/zmieszaniu:
Roztwory w komorach A (250 ml) i B (4750 ml) sg mieszane w celu otrzymania jednego odtworzonego/ zmieszanego roztworu (5000 ml) skfadajqcego sip z:
Wapn, Ca:~ Magnez, Mg:’ Sod. Na‘ Chlorki, Cl-Mleczan Wodoroweglan, Potas, K6 Glukoza
Teoretyczna osinolarnosc: 297 mOsm/l inne sktadniki leku to: dwutlenek wegla, woda do wstnzykiwan. pH odtworzonego/zmieszanego roztworu wynosi: 7 0 do 8,5
JAK WYGLADA
PRISMASOL 2 MMOL/L POTASU I CO ZAWIERA OPAKOWANIE
Prismasol 2 mmol/l potasu jest pakowany w dwukomorowe worki zawierajace w mniejszej komorze A roztwor elektrolitow, a w wiekszej komorze B - roztwor bufcrowy. Ostateczny odtworzony/zmieszany roztwor uzyskuje po rozerwa-niu spawu i wymieszania obu roztworow. Odtworzony/zmieszany roztwor jest przezroczysty i lekko zolty. Kazdy worek (A+B) zawiera 5000 ml roztworu do hemofiltracji i hemodializy. Kazdy worek jest umieszczony w przezroczystym opakowaniu zewnptrznym.
W kazdym opakowaniu znajduja sie dwa worki i ulotka informacyjna,
PODMIOT ODPOWIEDZIALNY I WYTWORCA:
Gambro Lundia AB Magistratsvagsn 16 SE-220 10 Lund SZWECJA
PRODUCENT:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO) WtOCHv
Data ostatniej aktualizacji ulotki: 07/2012
The following information is intended for healthcare professionals only
Prismasol® 2 mmol/l Potassium Solution for haemodialysis/ haemofiltration
PRECAUTIONS:
Carefully follow the instructions for use/handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock.
METHOD OF ADMINISTRATION:
Intravenous use and for haemodialysis. Prismasol, when used as a
substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (postdilution).
POSOLOGY;
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and
adolescents: 500 - 3000 ml/hour Children: 15-35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and
adolescents: 500 - 2500 ml/hour Children: 15-30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 I.
INSTRUCTIONS FOR HANDLING:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged.
Ail seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no
longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/ or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5) Medication should only be added to the solution under the responsibility of a physician In the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
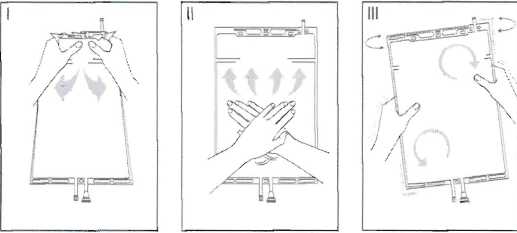
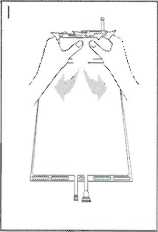
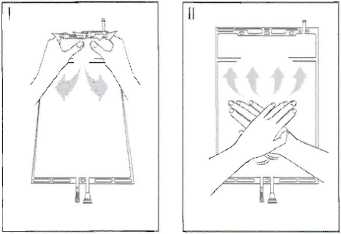
I Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments, (See figure I below)
II Push with both hands on the large compartment until the peel seal between the too compartments is entirely open. (See figure II below)
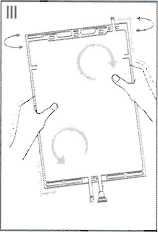
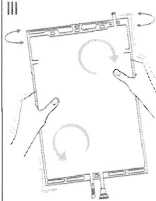
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
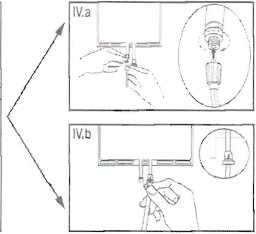
IV The dialysis or replacement line may be connected to either of the too access ports.
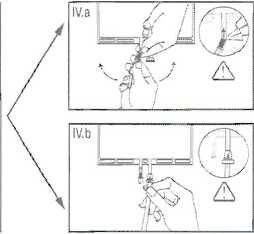
IV.a If the luer access is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure tV.a below),
When the dialysis or replacement line is disconnected from the iuer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
IV.blf the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-
renal replacement equipment.
STORAGE PRECAUTIONS:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
Az alabbi informaciok kizarolag egeszsegugyi szakembereknek szolnak
Prismasob 2 mmol/l kalium Hemodializalo vagy hemofiltracios oldat
OVINTEZKEDESEK:
Gondosan kovesse a hasznaiati es kezelesi utasitast.
Felhasznalas elott az elektrolitoldatot ossze kell kevemi a pufferoldattal. hogy letrejojjdn az aikalmazasra kesz oldat, amely alkalmas a hemofiltraciora, a hemodiafiltraciora vagy a folyama-tos hemodializisre.
Az oldat testhomersekletre (+37°C) valo felmeleg'itesekor gondosan allenorizni kell, hogy az oldat tiszta-saga megorzodott-e szemcsekep-zodes nelkul.
A kaliumszintet szorosan nyomon kell kovetni a legmegfelelobb kali-umkoncentracid kivalasztasahoz.
A szervetlen foszfat koncentra-ciojat rendszeresen merni kell. A szervetlen foszfatot potolni kell olyan esetekben, amikor a verben alacsony a foszfatszint.
A folyadekegyensuly zavarai eseten a klinikai allapotot gondosan nyomon kell kovetni, es a folyadek-egyensuiyt helyre kell aliitani, Szennyezett hemofiltracios es hemodializalo oldat alkalmazasa vermergezest es sokkot okozhat.
ALKALW1A2AS WIODJA:
Intravcnaa alkalmazasra es
hemodializishez, Potlo oldatkent a Prismasolt beadhatjak a korbe a hemofiltracio elott (predilucio) vagy utan (posztdilucio).
ADAGOLAS:
A Prismasol alkalmazando mennyi-sege a beteg klinikai allapotatol es az elerni kivant folyadekegyensuly-tol fugg, Az alkalmazando meny-nyiseget ezert a kezelesert felelos on/os megftelese hatarozza meg,
A pot!6 oldat lehetseges aram-lasi sebessegei hemofiltracio es hemodiafiltracio eseten az alabbiak:
Felnotteknel es
serdilloknel: 500 - 3000 ml/6ra
Gyermekeknel: 15-35 ml/ttkg/ora A aializald oldat (dializatum) lehetseges aramlasi sebessegei folyamatos hemodializis es folya-matos hemodiafiltracio eseten az alabbiak:
Felnotteknel es
serdUloknel: 500 - 2500 ml/6ra
Gyermekeknel: 15-30 ml/ttkg/ora FelnStteknel attaiaban a 2000 ml/ora koruli aramlasi sebes-seget hasznaljak, ami megkozeli-toleg napi 55 Uteres mennyisegnek felel meg,
KEZELESI UTASiTAS:
Az oldat ketrekeszes zsak kiszere-lesben kerul forgalomba.
A betegnek valo adagolas soran vegig aszeptikus modon kell eljarni Kizarolag akkor hasznalja fel, ha az oldat tiszta, valamint sertetien a kulso csomagolaa. Minden leza-
rasnak sertetlennek kell lennie. Ha szivargast eszlel, azonnal dobja ki az oldatot, mivel a sterilitas tdbbe mar nem garantalhato.
A nagyobbik (B) rekeszhez egy injekcios bemenet tartozik. szuk-seg eseten ebbe lehet adagolni tovabbi gyagyszereket az oldat bsszeallitasa uian, Akezelfiorvos felelossege annak megitelese, hogy a Prismasol oldattal egyutt beadott gyogyszer kompatibilis-e az oldattal; emellett ellenoriznie kell a szinvaltozast es/vagy a kicsapo-das, illetve oldhatatlan komplexek es kristalyok kepzddesenek jeleit, Nezze at a hozzaadni kivant gyogyszer Alkalmazasl eldirasat.
Egyeb gyogyszer hozzaadasa elott ellenorizze, hogy az a Prismasol oldat pH-ertekenek megfelelo vizes kbzeaben oldhatb es stabil-e (a fel-hasznalasra kesz oldat pH-erteke 7.0 -8,5).
Az oldathoz kizarolag a kezelo-orvos felelossegere lehet tovabbi gyogyszereket adni, a kcvetkezo modon: Tavolitson el minden folya-dekot az injekcios bemenetbol, tart-sa fejjel lefele a zsakot, vigye be a gyogyszert az injekcios bemeneten kereszti.il, majd alaposan keverje ossze az oldatot. A kesz oldatot azonnal be kell adni.
( Kozvetlenul a felhasznalas elott vegye le a zsakrol a kulso csomagolast. es keverje ossze a ket rekesz tartalmat. A kiseb-bik rekeszt mindket kezevel tartva preselje addig. amig mec nem nyilik benne az atjaras a ket rekesz kozott. (Lasd az alabbi I. abrat.)
(I Nyomja ossze a nagyobbik rekeszt mindket kezevel addig. amig teljesen meg nem nyilik a lezaras a ket rekesz kozott. (Lasd az alabbi II. abrat.)
III Ugyeljen az oldat biztos ossze-keveredesere, razzafel ovato-san a zsakot, Az oldat most mar keszen all a felhasznalas-ra, es felakaszthatja a keszu-lekre. (Lasd az alabbi III. abrat,)
IV A dializalo vezeteK vagy a potlo oldat vezeteke a ket bemeneti nyllas barmelyikehez csatla-koztathato.
IV.a Ha a Luer-csatlakozos beme-netet hasznalja, forgato es huzo mozdulattal vegye le a kupakot, es forgato es nyomo mozdulattal csatlakoztassa a dializalo vagy a potlo oldat ve-zetekenek apavegu Luer-zarjat a zsakon levo anyavegu Luer-aljzatba. Ellenonzze, hogy a csatlakozas megfelelo-e, es szoritsa meg. A csatlakozo most nyitva van. Ellenorizze. hogy a folyadek szabadon aramlik-e. (Lasd az alabbi IV.a abrat.)
Ha a dializalo oldat vagy a pot-16 oldat vezeteket levalasztjak a Luer-csatlakozorol a csatlakozo elzarodik. es a folyadek aramlasa megszunik. A Luer-csatlakozo tumentes es tisztit-hato csatlakozo.
IV.bHa az injekcios bemenetet hasznalja, eloszor vegye le a vedosapkat. Ezutan szurja at a tiisket a gumimembranon, Ellenorizze, hogy a folyadek szabadon aramlik-e. (Lasd az alabbi IV.b abrat)
Kizarolag megfelelo extrarenalis vesepotlo berendezesse! hasznal-hato.
KULONLEGESTAROLASI ELOiRASOK:
Az alkalmazasra elokeszitett oldat hasznalat kozbeni kemiai es fizikai stabilitasat 24 oran at vizsgaltak +22'C-on. Kemiai tenyezok miatt az osszeallltott oldatot azonnal fel kell hasznalni. Ha a kesz oldatot nem hasznaljak fel azonnal, az oldat tarolasi idejeert es korCilme-nyeiert a felhasznalo felelos; a kesz oldat tarolasa ne tartson tovdbb 24 oranal. beleertve a kezeles iddtartamat is.
A kesz oldat kizarolag egyszeri felhasznalasra szolgal. A fel nem hasznalt oldatot az alkalmazas befejezese utan azonnal dobja el.
Informacje przeznaczone wyt^cznie dla fachowego personelu medycznego
PrismasoF 2 mmol/i potasu Roztwor do hemodializy/ hemofiltracji
SRODKI OSTROZNOSCI:
DoKladnie przestrzegac instrukcji uzycia/pcst^powania.
Roztwor elektrolitow musi zostac zmieszany z roztworem bufo-rowym przed uzyciem w celu uzyskania koncowego roztworu do hemofiltracji/hemodiafiltracj! lub ci^gtej hemodializy.
Nalezy starannie kontrolowac ogrzewanie roztworu do temperatu-ry data (+37' C), sprawdzaj^c, czy roztwor jest przezroczysty i wolny od czastek statych.
Nalezy doktadnie monitorowac kaliemie w celu umozliwienia prawidtowego wyboru najodpowied-niejszego stezenia potasu.
Nalezy regularnie mierzyc st^zenie nieorganicznych fosforandw. W przypadku hipofcsfatemii (niskie-go stezenia fosforandw we krwi), nieorganiczne fosforany nalezy uzupetniac
W przypadku braku rownowagl ply-now naleiy starannie monitorowac stan kliniczny pacjenta i przywracac normalny bilans ptynow.
Uzycie zanieozyszczonych roztwo-rdw do hemofiltracji i hemodializy nnoze spowodowac posocznic§ i wstrzas.
SPOSOB PODAWANIA:
Do podawania dozylnego i hemodializy. Prismasol 2 mmot/l potasu uzywany jako roztwor zamienny jest podawany do obwodu przed hemofiltrem (predylucja) lub za hemofiltrem (postdylucja).
DAWKOWANIE:
Obj^tosc uzywanego roztworu Prismasol 2 mmol/l potasu zalezy od stanu klinicznego pacjenta i docelowego bilansu ptynow. Diatego decyzj§ o obj^tosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Szybkosci przeptywu dla roztworu zamiennego w hemofiltracji i hemo-diafiitracji wynosza:
Dorosli i
mtodziez: 500 do 3000 ml/godz.
Dzieci: 15 do 35 ml/kg mc./godz.
Szybkosci przeptywu dla roztworu do dializy (dializat) w ciagtej he-modializie i cipgtej hemodiafiltracji wynosza:
Dorosli i
mtodziez: 500 do 2500 ml/godz.
Dzieci: 15 do 30 mj/kg mc./godz. Najczesciej stosowane szybkosci przeptywu w przypadku dorosiych wynosza okoto 2000 ml/godz,. co odpowiada dziennej obj^tosci wynoszacej 55 I.
INSTRUKCJA POST^POWANIA:
Roztwor jest pakowany w dwuko-morowe worki.
Podczas podawania leku pacjen-towi nalezy stosowao technik? aseptyczna.
Uzywac wytcjoznie wtedy, gdy roztwor jest przezroczysty, a
rewn^trzne opakowanie nieusz-
kodzone. Wszystkie spawy musza bye nienaruszone. W przypadku zauwazenia przecieku roztwor nalezy niezwtocznie wyrzucic, po-niewaz nie mozna zagwarantowac jatowosci.
Duza komora B wyposazona jestw port do wstrzykni^c, umozliwiaja.cy dodanie po odtworzeniu/zmiesza-niu do roztworu innych koniecznych lekdw. Lekarz pozostaje odpowiedzialny za okreslenie niezgodnosci lekdw dodatkowych z roztworem Prismasol za pomoca sprawdzania zmiany koloru i (lub) wytwarza-nia osadu, nierozpuszczalnych kompleksow lub krysztalow. Nalezy zapoznac sie z instrukeja uzyeia dodawanego leku.
Przed dodaniem leku nalezy sprawdzic, czy jest rozpuszczalny i stabilny w wodzie o pH takim jak roztwor Prismasol 2 mmol/l potasu (pH odtworzonego/zmieszanega roztworu wynosi od 7,0 do 8,5).
Leki powinny bye dodawane do roztworu pod kontrola. lekarza w nastepujgcy sposob: Usunac plyn z porui do wstrzykniec, przytrzymac worek do gory nogaml. wstrzyknac lek przez port i starannie wymie-szac. Roztwor nalezy podac niezwtocznie.
I Bezposrednio przez uzyciem zdjgc zewngtrzne opakowanie z worka i wymieszac roztwory z dwoch roznych komor. Uchwy-cic matg komore dtorimi i sci-sngc do momentu powstania otworu w razrywalnym spawie oddzielajgcym obydwie komo-ry. (Patrz rysunek ! ponizej),
I! Obiema dloiirni nacisngc duzg komorp do momentu catkowite-go otwarcia rozrywalnego spa-wu pomiedzy dvvoma komora-mi. (Patrz rysunek II ponizej).
III Catkowicie wymieszac roztwor, wstrzgsajac deiikatnie worek. Roztwor jest teraz gotowy do uzycia, a worek mozna zawie-sic na stojaku. (Patrz rysunek III ponizej).
IV Do kazdego z dwoch portow dostepu mozna podigczyc iinig dializy lub wymiany.
IV.a Jesli korzysta sie z dostppu typu Luer, usungc zatyczkg, ocikrecajac jg i pociagajgc, a nastepnie podigczyc mgskg koricowke Luer Lock linii dializy lub wymiany do zenskiej koh-cowki na worku, wkrecajac ja i wciskaja.c. Upewnic sie, ze po-Igczenie jest wystarczajgco mocne. Teraz port jest otwarty. Sprawdzic, czy ptyn przeplywa swobodnie, (Patrz rysunek IV.a ponizej).
Gdy llnie dializy iub wymiany bedg odtaczone od ztgcza typu Luer. potaczenie zostanie za-mkniete i przeptyw ptynu wstrzyniany. Port typu Luer jest bezigtowy i ma mozliwosc pod-taczania dodatkowych przepty-wow w czasie dziatania.
IV.bW przypadku korzystania z portu do wstrzykniec najpierw naiezy zdjac kapsel. Nastgpnie wprowadzic grot przez gumowg przegrode. Sprawdzic. czy ptyn przeplywa swobodnie. (Patrz rysunek IV.b ponizej).
SRODKI 0STR02N0SCIPRZY PRZECHOWYWANIU:
Wykazano chemiczng i fizyczna stabilnobd odtworzonego roztworu w ciggu 24 godzin w temperaturze +22' C. Z chemicznego punktu widzenia odtworzony roztwor powinien zostac niezwlocznie uzyty. Jesli nie zostanie zuzyty od razu. za czas i warunki przechowy-wania przed uzyciem odpowiada uzytkownik i normalnie czas ten nie powinien przekraczac 24 godzin lacznie z czasem zabiegu. Odtworzony roztwor nie nadaje sip do powiornego uzycia. Usunac caty niezuzyty roztwor bezposrednio po uzyciu.
Naiezy uzywac wytgcznie z odpo-wiednimi urzgdzeniami do wymiany pozanerkowej.

|
IV.a |
/I "h\ | / \ i x ! ^ 1© \ u J |
|
IV.b |
© _......_ PQx© |
THIS PAGE IS INTENTIONALLY LEFT BLANK

o
<
w
TOO
‘ o E !£8
Prismasol and Gambro are trademarks belonging to the Gambro Group
id O o 5 aia;N s
£ o C' S ro v-> lu 3
O Q_ GO CO
ro^ E
|>f 3>
lei
Prismasof 2
UK, IE, WIT Package leaflet: Information for the user.............................3
HU Betegtajekoztato: Informaciok a felhasznalo szamara........5
PL Ulotka dla pacjenta..............................................................7
UK, IE, WIT The following information is intended
for healthcare professionals only.........................................9
HU Az alabbi informaciok kizarolag
egeszsegugyi szakembereknek szolnak...........................11
PL Informacje przeznaczone wytacznie
dla fachowego personelu medycznego.................. 13
DGS7250G1 Rev. 2013-03 4718
fc,GAMBRO
THIS PAGE IS INTENTIONALLY LEFT BLANK
Package leaflet: Information for the user
Prismasol' 2 mmol/l Potassium Solution for haemodialysis/ haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read alt of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
WHAT IS IN THIS LEAFLET:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. WHAT PRISMASOL IS AND WHAT IT IS USED FOR
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiitra-tion (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable orfilterabie substances. Prismasol 2 mmol/l Potassium is indicated particularly in patients who have tendency to hyperkalaemia (a high concentration of potassium in the blood).
2. WHAT YOU NEED TO KNOW BEFORE YOU ARE GIVEN PRISMASOL 7 8
OTHER MEDICINES AND PRISMASOL
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood),
• Vitamin D and medicinal products containing calcium as they can increase the risk of hyper-calcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
PREGNANCY AND BREASTFEEDING
If you are pregnant or breastfeeding, tniriK you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. As ail medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
DRIVING AND USING MACHINES
Prismasol is not known to affect your ability to drive or use machines.
3. HOW PRISMASOL IS USED
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend ori your clinical condition
|
mmol/l |
mEq/l | |
|
Calcium, Ca:8 |
1.75 |
3.50 |
|
Magnesium. Mg-" |
0.50 |
1.00 |
|
Sodium, Na8 |
140.00 |
140.00 |
|
Chloride. Cl |
111,50 |
111.50 |
|
Lactate |
3.00 |
3 00 |
|
Hydrogen carbonate, HCO.y |
32.00 |
32.00 |
|
Potassium, K8 |
2.00 |
2.00 |
|
Glucose |
6.10 |
and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor. Administration route: Intravenous use and for haemodialysis,
For instructions for use, please see section 'The following information is intended for healthcare professionals only”.
IF YOU THINK YOU ARE GIVEN MORE PRISMASOL THAN YOU THINK YOU SHOULD BE
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure. Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hy-pophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration). Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. HOWTO STORE
PRISMASOL_
Keep this medicine out of the sight and reach of children.
Do not store below +4:C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6, CONTENTS OF THE PACK AND OTHER INFORMATION
WHAT PRISMASOL CONTAINS The active substances are: Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A))
contains
Calcium chloride dihydrate 5.145 g Magnesium chloride hexahydrate 2.033 g
1000 ml of buffer solution (from the farge compartment (B)) contains Sodium chloride 6.450 g
Sodium hydrogen
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
Theoretical Osmolarity:
297 mOsm/l
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution:
7.0-8.5
WHAT PRISMASOL LOOKS LIKE AND CONTENTS OF THE PACK
Prismasol is presented in a two-compartment bag containing in the smaller compartment/1., the electrolyte solution, and in the larger compartment B, the buffer solution, The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is dear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is oveavrapped with a transparent film.
Each box contains two bags and a package leaflet.
MARKETING AUTHORISATION HOLDER:
Gambro Lundia AB Magistratsvagen 16 SE-220 10 Lund SWEDEN
MANUFACTURER:
Gambro Dasco S.p.A Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012
Betegtajekoztato: Informaciok a felhasznalo szamara
Prismasol" 2 mmol/l kalium Hemodializalo vagy hemofiltracios oldat
Kalcium-klorid-dihidrat/ Magnezium-klorid-hexahidrat/ Glukoz-monohidrat/ 90% terfogat-szazalekos tejsavoldat/ Natrium-klorid/ Kalium-klorid/ Natrium-hidrogen-karbonat Mielott beadnak Qnnek ezt a gyogyszert, olvassa el figyelmesen az alabbi betegtajekoztatot, meiy az On szamara fontos informaciokat tartalmaz,
• Tartsa meg a betegtajekoz-tatot, mert a benne szereplo informacjokra a kesdbbiekben is sziiksege lehet.
• Tovabbi kerdeseivei forduljon ke-zeloorvosdhoz, gyogyszeresze-hez vagy a szakszemelyzethez.
• Ha Onnel barmilyen mellekha-tas jelentkezik, tajekoztassa errol kezeloorvosat, gyogysze-reszet vagy a szakszemelyze-tet. Ez a betegtajekoztatoban fel nem sorolt barmilyen lehetseges mellekhatasra is vonatkozik.
A BETEGTAJEKOZTATO TARTALMA:
1. Milyen tipusu gyogyszer a Prismasol es milyen betegsegek eseten alkaimazhato?
2. Tudnivalok a Prismasol alkalmazasa elott
3. Hogyan kell alkalmazni a Prismasolt?
4. Lehetseges mellekhatasok
5. Hogyan kell a Prismasolt tarolni?
6. A csomagolas tartalma es egyeb informaciok
1, MILYEN TIPUSU
GYOGYSZER A PRISMASOL ES MILYEN BETEGSEGEK ESETEN ALKALMAZHATO?
A Prismasol a kovetkezo hatd-anyagokat tartalmazza: kalcium-klorid-dihidrat, magnezium-klorid-hexahidrat, glukoz-monohidrat.
90% terfogat-szazalekos tejsavoi-dat, natrium-klorid, kalium-klorid es natrium-hidrogen-karbonat.
A Prismasol a veseelegte-lensegek kezeleseben alkal-mazott hemofiltracios vagy hemodiafiltracios oldat (a szuron at-halado verbol fellepo folyadekvesz-teseg pdtlasara), illetve folyamatos hemodializis vagy hemodiafiltracio soran alkalmazott oldat (amikor is a ver a dializald membran egyik oldalan aramiik. a hemodializalo oldat pedig a masikon),
A Prismasol oldat alkaimazhato dializalhato vagy szurheto anya-goktol elszenvedett gyogyszermer-gezes kezeleseben is.
A Prismasol 2 mmol/l kalium alkalmazasa kulonosen hiperkalemiara hajlamos betegeknel javallott (olyan betegeknel, akiknel fennall annak kockazata, hogy a ver tulzottan magas kaliumszintje alakuljon ki).
2, TUDNIVALOK A PRISMASOL ALKALMAZASA ELOTT 9 10
hemodializissel torteno kezeleseben jartas orvos altal szeme-lyesen - vagy az 6 felugyeletevel - alkaimazhato.
A kezeles elott es alatt ellenorzik a ver parametereit, pi. a sav-bazis haztartast es a verben az elektroli-tokat (a sok koncentraciojat),
A vercukorszintet folyamatosan monitorozni kell, kulonosen cukor-betegek eseteben.
EGYEB GYOGYSZEREK ES A PRISMASOL
Feltetlenul tajekoztassa kezeloorvosat vagy gyogyszereszet a jelen-leg vagy nemregiben alkalmazott, valamint alkalmazni tervezett egyeb gyoayszereirol,
Ez azert fontos, mert egyes gyogy-szerek koncentracidja csokkenhet a verben a kezeles hatasara, Kezeloorvosa eldonti majd. hogy szukseges-e gyogyszeres kezele-senek barmilyen modositasa. Kulonosen arrol fontos tajekoztat-nia kezeloorvosat, ha a kovetkezo gyogyszerek valamelyiket szedi:
• Digitalisz gybgyszerek (kulon-bozo sziv-rendelienessegek kezelesere), mivel a digitalisz gyogyszerek altal keltett aritmia (szabalytalan ritmusu vagy tul gyors szlweres) kockazata megno hipokalemias allapo-toknal (amikor alacsony a ver
kaliumkancentr&cipjci).
• D-vitamin es kalciumot tar-talmazo gyogyaszati keszit-menyek, mivel ezek novelik a hiperkalcemia (a ver megnovekedett kalciumkoncentracioja) kockazatat.
• Egyeb olyan gyogyszerek, amelyek natrium-hidrogen-karbonatot is tartalmaznak, mert ezek alkalmazasa eseten megno a metabolikus alkalozis (tulzott bikarbonatmennylseg a verben) kockazata.
TERHESSEG ES SZOPTATAS
Ha On terhes vagy szoptat, illetve ha fennall Onnel a terhesseg lehe-tosege vagy gyermeket szeretne, a gyogyszer alkalmazasa elott beszeljen kezeloorvosaval vagy gyogyszereszevel.
Amennyiben On terhes vagy szoptat, minden egyeb gyogyszerhez hasonldan kezeloorvosa donti el, hogy kaphat-e Prismasolt vagy sem.
A KESZITMENY HATASAI A GEPJARMUVEZETESHEZ ES GEPEK KEZELESEHEZ SZUKSEGES KEPESSEGEKRE
A Prisfnasol eseteben nem allapi-tottak meg, hogy hatassal lenne a gepjarmuvezeteshez es aepek keze-lesehez szukseges kepessegekre.
3. HOGYAN KELL ALKALMAZNI A PRISIVIASOLT?_
A gyogyszert mindig a kezeloorvosa vagy gyogyszeresze altal elmon-dottaknak megfeleloen alkalmazza, Amennyiben nem biztos az adago-last illetoen, kerdezze meg kezelo-orvosat vagy gyogyszereszet.
A Prismasol alkalmazando meny-nyisege a beteg klinikai allapotatol es az elerni kivant folyadekegyen-sulytol fugg. Az alkalmazando mennyiseget ezert a kezeloorvos megitelese hatarozza meg.
Az alkalrnazas modja: Intravenas alkalmazasra es hemodializishez,
A hasznalati utmutatot lasd ,.Az alabbi informaciok kizarolag egesz-segugyi szakembereknek szolnak’' cimu reszben.
HA AZ ELOIRTNAL TOBB PRISIVIASOLT KAPOTT
Az eljaras ideje alatt gondosan monitorozzak a folyadekegyensulyt. valamint az elektrolit- es a sav-ba-zis egyensulyt,
A tuladagolas folyadekterheleshez vezethet, ha veseelegtelensegben szenved.
A tuladagolas sulyos kovetkezme-nyeket okozhat, mint a panaasos szivelegtelenseg, illetve az elektrolit- es sav-bazis zavarok,
A hemofiliracio folyamatos alkal-mazasa megszunteti a folyadek-es elektrolitfelesleget. Folyadek tultoltes sseten az ultrafiltraciot nbveln kell, tovabba csbkkenteni kell a hemofiltracios oldat adagolasanak se-bessegei, Sulyos kiszaradas eseten az ultrafiltraciot el kell hagyni, illetve megfeleio mertekben nbvelni kell a hemofiltracios oldat bearamlasat.
Ha barmilyen tovabbi kerdese van a gyogyszer alkalmazasaval kapcsolatban, kerdezze meg keze-loon/osat, gyogyszereszet vagy a szakszemelyzetet.
4. LEHETSEGES MELLEKHATASOK
Mint minden gyogyszer, Igy ez a gyogyszer is okozhat mellekha-tasokat, amelyek azonban nem mindenkinel jelentkeznek,
Az alabbi nemkivanatos hatasok azonban elkepzelhetok lehetnek az oldat alkalmazasakor: A viz korosan magas vagy alacsony mennyisege a testben (hiper- vagy hipohidracid), a verben oldott sok zavarai (elektrolitzavarok), a foszfatok rendellenesen alacsony koncentracioja a verben (hipofoszfatemia), korosan magas vercukorszint (hiperglikemia) a ver vegyhatasanak lugos iranyu eltolodasa (metabolikus alkalozis, a plazma bikarbonat-koncentracioja-nak megemelkedeseert elsoalege-sen feleibs folyamat).
A aializiskezelesekkel kapcsolatban fellephet nehany nemkivanatos hatas, mint peldaul nanyinger (emelyges), hanyas (rosszullet), izomgdrcsbk es alacsony vernyo-mas (hipotenzio).
Ha Onnei barmilyen mellekhatas jelentkezik tajekoztassa kezeloor-.'o-sat, gyogyszereszet vagy a szakszemelyzetet, Ez a betegtajekoztatoban fel nem sorolt barmilyen lehetseges mellekhatasra is vonatkozik.
5. HOGYAN KELL A
PRISIVIASOLT TAROLNI?
A gyogyszer gyermekektol elzarva tartando!
Ne tarolja +4°C alatti homersek-leten.
A cimken es a csomagolason feltuntetett lejarati ido utan ne alkalmazza ezt a gyogyszert. A lejarati ido a megadott honao utolso napjara vonatkozik.
6. A CSOMAGOLAS TARTALMA ES EGYEB INFORMACIOK
MIT TARTALMAZ A PRISMASOL A keszitmeny hatoanyagai: Osszekeveres elatt:
A kisebbik (A) rekeszben levo elektrolitoldat tartalma 1000 ml-re vetitve:
Kalcium-klorid-dihidrat 5 145 g
Magriezium-klorid-hexahidrat 2 033 g
A nagyobbik (B) rekeszben levo pufferoldat tartalma 1000 ml-re vetitve:
Natrium-hidrogen-karbonat 3,090 g
Osszekeveres utan:
Az A rekeszben levo ole at (250 ml) es a B rekeszben levo oldat (4750 ml) osszekeverese utan a vegleges oldat (5000 ml) tartalma:
|
mmol/l |
mEq/I | |
|
Kaicium, Ca |
1 75 |
3 50 |
|
Magnezium, Mg:10 |
0,50 |
1.00 |
|
Natrium, Na“ |
140,00 |
140.00 |
|
Klorid, Cl |
111,50 |
111,50 |
|
Laktat |
3.00 |
3,00 |
|
Hidrogen-karbonat, HCO |
32 00 |
32,00 |
|
Kalium, K10 |
2,00 |
2.00 |
|
Glukoz |
6,10 |
Elmeleti ozmolaritas: 297 mOsm/1 Egyeb osszetevok: szen-dioxid, Illetve injekeiohoz valo viz Az osszekevert oldat kemhatasa (pH), 7,0-8,5
MILYEN A PRISMASOL KESZITMENY KULLEME ES MIT TARTALMAZ A CSOMAGOLAS
A Prismasol ketrekeszes zsak formatumu kiszerelesben kerul forgalomba; a kisebbik (A) rekesz tartalmazza az elektrolitoldatot, a nagyobbik (B) rekesz pedig a pufferoldatot. Az alkalmazasra elokeszftett, vegleges oldat a felnyithato lezaras szettorese (nyi-tasa) utan a ket oldat osszekevere-desevel jon letre, Az alkalmazasra kesz oldat tiszta es enyhen sargas szinii. A ket zsak (A+B) 5000 ml oldatot tartalmaz hemofiltraciohoz es hemodializishez, A zsak ailatszo fdiiaval is be van csomagolva. Mindegyik doboz ketzsakot es egy betegtajekoztatot tartalmaz,
A FORGALOMBA HOZATAL! ENGEDELY JOGOSULTJA:
Gambro Lundia AB Magistratsvagen 16 SE-220 10 Lund SVEDORSZAG
GYARTO:
Gambro Dasco S.p.A,
Sondalo Plant Via Stelvio S4 23035 Sondalo (SO) OLASZORSZAG
OGYi-T-21178/05 2 x 5000 ml tobbretegii poliolefin anyagbol keszult ketrekeszes (A es B) zsakba toltve, szeleppel.
A betegtaj^koztato legutobbi felulvizsgalatanak datuma:
2012 oktdber
Ulotka dla pacjenta
PrismasoF 2 mmol/l potasu Roztwor do hemodlalizy/ hemofiltracji
Wapnia chlorek dwuwodny/ magne-zu chlorek szesciowodny/ glukoza jednowodna/ kwasu mlekowego roztwor 90% w/w / sodti chlorek,' potasu chlorek/ sodu wodorowe-glan
Nalezy zapoznac si? z trescl? ulotki przed przyjeciem leku, poniewaz zawiera ona informacje wazne dla pacjenta,
• Nalezy zachowac t? ulotk?, aby w razie potrzeby mocja ponow-nie przeczytac,
• Nalezy zwrocic sie do lekarza, farmaceuty lub pielegniarki w razie jakichkolwiek dalszych watpliwosci.
• Jesli nasili sie ktorykolwiek z objawow niepozadanych lub vvystqpia jakiekolwiek objawy niepozadane. w tym niewy-mienione w ulotce, nalezy powiedziec o tym lekarzowi, farmaceucie lub pielegniarce.
SPIS TRESCi ULOTKI:
1 Co to jest Prismasol 2 mmol/l potasu i w jakim celu sie go stosuje
2. Informacje wazne przed przyjeciem roztworu Prismasol 2 mmol/l potasu
3. Jak stosowac Prismasol 2 mmol/l potasu
4. Mozliwe dziatania niepozadane
5. Jak przechowywac Prismasol 2 mmol/l potasu
8. Zawartosc opakowania i Inne informacje
1, CO TO JEST
PRISMASOL 2 MMOL/L POTASU I W JAKIM CELU SI5 GO STOSUJE_
Prismasol zawiera substancje czynne: wapnia chlorek dwuwodny. magnezu chlorek szesciowodny.. glukoza jednowodna, kwasu mlekowego roztwor 90% w/w, sodu chlorek. potasu chlorek. sodu wodoroweglan.
Prismasol 2 mmol/l potasu jest sto-sowany w leczeniu niewydolnosci nerek jako roztwor do hemofiltracji lub heinodiafiltracji (jako roztwor zast?pczy przy utracie piynow z krwi przeplywajacej przez filtr) oraz ciaglej hemodializy lub hemodia-filtracji (krew przeptywa po jednej stronie membrany dializacyjnej, podczas gdy roztwor do hemodializy przeptywa po drugiej stronie membrany).
Prismasol 2 mmol/l potasu mozna rowniez stosowac w przypadku zatrucia lekami zawierajacymi substancje ulegajace dializie lub filtracji.
Prismasol 2 mmol/l potasu jest wskazany szczegolnie u pacjentow, majacych sktonnosc do hiperkalie-mii (wysokie steZenie potasu we krwi).
2. INFORMACJE WAZNE PRZED PRZYJECIEM ROZTWORU PRISMASOL 2 MMOUL POTASU 11 12
Roztwor powinien bye uzywany wyt?cznie przez lub pod nadzo-rem lekarza wykwalifikowanego w leczeniu niewydolnosci nerek przez zastosowanie hemofiltracji, hemo-diafiltracji i ciggiej hemodializy, Przed zastosowaniem leczenia i podczas niego zostanie zbadana krew pacjenta. np, rdwnowaga kwasowo-zasadowa i stezenie elektrolitow (soli we krwi).
NaleZy dokladnie monitorowac stezenie glukozy we krwi, zwfaszcza gdy paejent choruje na cukrzyc?.
INNE LEKI I
PRISMASOL 2 MMOL/L POTASU
Nalezy powiedziec lekarzowi lub farmaceucie o wszystkich lekacb przyjmowanych obecnie lub ostat-nio a takze o lekach, ktdre paejent planuje przyjmowac.
Stezenie we knvi Innych przyjmowanych lekow moze zostac obnizone podczas leczenia. Lekarz zdecyduje. czy wymagana jest zmiana przyjmowanych lekow.
W szozegolnosci nalezy powiedziec lekarzowi o przyjmowaniu nastepu-jpcych lekow:
• glikozydow (stosowanych w leczeniu okreslonych chordb serca), gdyz podczas hipokalle-mii (obnizonego stezenia potasu we krwi) zwi?kszaja one ryzyko wystapienia zaburzeri rytmu serca (nieregularnego lub przy-spieszonego bicia serca);
• witaminy D i produktow lecz-niezyeh zawierajpcych vvapri, gdyz mog? one zwiekszac ryzyko wystapienia hiperkalce-mii (wysokiego stezenia wapnia we krwi);
• jakichkolwiek dodatkow wodoroweglanu sodu obecnych w innych lekach. gdyz moze on zwi?kszac ryzyko wystapienia zasadowicy metabolieznej (nadmiaru wodoroweglanow we knvi).
CIAZA I KARMIENIE PIERSIA
W ciazy i w okresie karmienia pier-si? lub gdy istnieje podejrzenie, ze kobieta jest w ciazy, lub gdy planuje ciaze. przed zastosowaniem tego ieku nalezy poradzie sip lekarza lub farmaceuty,
Tak jak w przypadku wszystkich lekow, iekarz zadecyduje o poda-
6. ZAWARTOSC OPAKOWANIA I INNE INFORMACJE_
CO ZAWIERA
PRISMASOL 2 MMOL/L POTASU Substancje czynne:
Przed odtworzeniem/zmiesza-niem:
1000 ml roztworu elektrolitowego
mmol/l mEg/l 1,75 ~ 3,50 0.50 1,00
140,00 140,00 111,50 111,50 3.00 3,00
waniu roztworu Prismasol kobietom w ciazy lub karmiacym piersig.
PROWADZENIE POJAZDOW t OBSLUGA MASZYN
Nie wiadomo, czy Prismasol wplywa na zdolnosc prowadzenia pojazdow i obstugi maszyn.
3. JAK STOSOWAC PRISMASOL 2 MMOL/L POTASU_
Ten lek naiezy zawsze stosowac zgodnie z zaleceniami lekarza, W razie wgtpliwosci naiezy zwrocic sie do lekarza lub farmaceuty.
Objgtosc uzywanego roztworu Prismasol 2 mmol/l potasu zalezy od stanu klinlcznego pacjenta i docelowego bilansu plynow. Dlatego decyzj^ o obj^tosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Droga podawania: do podawania dozylnego i hemodiallzy.
Instrukoje uzycia znajduja sip w czgsci „lnformacje przeznaczone wytacznie dla fachowego personelu medycznego".
GDY PACJENTOW1 WYDAJE SIE, ZE PODANO MU WI^KSZA ILOSC ROZTWORU PRISMASOL NIZ ZALECANA
Naiezy dokladnie monitorowac bilans plynow, rownowage elektroli-towa i kwasowo-zasadowa. Przedawkowanie moze spowodo-wac przeciazenie ptynami u pacjen -tbvv z niewydolnoscia nerek. Przedawkowanie moZe prowadzIC do powaznych nastgpstw, takich jak zastoinowa niewydolnosc serca, zaburzenia rownowagi elektrolito-wej czy kwasowo-zasadowej.
Giggte stosowanie hemoflltracji usuwa nadmiar plynu i elektrolitow. W przypadku przewodnienia naiezy zwiekszyc ultrafiltracjg i zmniejszyc wielkosc przeptywu roztworu do hemoflltracji. W przypadku cigz-kiego odwodnienia konieczne jest przerwanie ultrafiliracji i odpowied-nie zwiekszenie naplywu roztworu do hemoflltracji.
W razie jakichkolwiek dalszych watpliwosci zwigzanych ze stoso-waniem tego leku naiezy zwrocic sie do lekarza, farmaceuty lub pielegniarki.
4. MOZLIWE DZIALANIA
NIEPOZAOANE_
Jak kazdy lek. lek ten moze powo-dowac dziatania niepozadane, cho-ciaz nie u kazdego one wystapia. Mozliwe s£( nastgpujqce dzialania niepozadane zwigzane z uzywa-niem roztworu: przewodnienie lub odwodnienie (nieprawidtowo wysoka lub niska objgtosc vvody w organizmie), zaburzenia elektro-litowe (sole mineralne we krwi), hipofosfatemia (nieprawidtowo niskie st^zenie fosforanow we krwi), hiperglikemia (nieprawidtowo wysokie stgzenie glukozy we krwi) i alkaloza metaboliczna (proces, ktory powoduje gtbwnie zwigksze-nie stezenia wodoroweglanu w osoczu).
Wloga wystgpic pewne reakcje niepozadane zwl^zane z dializa, np. nudnosci, wymioty, skurcze miesni oraz niedocisnienie (niskie cisnienie krwi).
Jesli wystapi^ jakiekolwiek objawy niepoza.dane, w tym wszelkie mozliwe objawy niepozgdane niewymienione w ulotce, naiezy zwrocic sie do lekarza, farmaceuty lub pielegniarki.
5. JAK PRZECHOWYWAC PRISMASOL 2 MMOL/L POTASU_
Lek naiezy przechowyvvac w miej-scu niewidocznym i niedostepnym dla dzieci.
Nie przechowyvvac w temperaturze ponizej +4=C.
Nie stosowac tego leku po uptywie terminu waznosci zamieszczonego na etykiecie i opakowaniu. Termin
waznosci oznacza ostatni dzion
danego miesiaca.
(mata komora A) zawiera:
Yv/apnia chlorek dwuwodny 5.145 g Magnezu chlorek
szesciowodny 2,033 g
Glukoza bezwodna 22.000 g
Kwas (S)-mlekowy 5,400 g
1000 ml roztworu buforowego (ciuza komora B) zawiera:
Sodu chlorek 6 450 g
Sodu wodoroweglan 3,090 g
Potasu chlorek 0,157 g
Po odtworzeniu/zmieszanlu:
Roztwory w komorach A (250 ml) i B (4750 ml) sg mieszane vv celu otrzymania jednego odtvvorzonego/ zmieszanego roztworu (5000 ml) sktadajgcego sie z:
Wapri, Ca:' Magnez, Mg:’ Sod, Na' Chlorki, Cl' Mleczan
Wodoroweglan, HCO.; 32.00 32.00 Potas, K12 ' 2,00 2,00
Glukoza 6,10
Teoretyczna osmolarnosc; 297 mbsm/l
Inne skfadnlki leku to: dwutlenek wegla, woda do wstrzykiwan. pH odtworzonego/zmieszanego roztworu wynosi: 7,0 do 8.5
JAK WYGLA)DA
PRISMASOL 2 MMOUL POTASU I CO ZAWIERA OPAKOWANIE
Prismasol 2 mmol/l potasu jest pakowany w dwukomorowe worki zawieraja.ee w-mniejszej komorze A roztwor elektrolitow, a w vvigksze.i komorze B - roztwor but'orowy. Ostateczny odtworzony/zmieszany roztwor uzyskuje po rozerwa-niu spawu i wymieszania obu roztworow. Odtworzony/zmieszany roztwor jest przezroczysty i lekko zolty. Kazdy worek (A+B) zawiera 5000 ml roztworu do hemoflltracji i hemodializy. Kazdy worek jest umieszczony w przezroczystym opakowaniu zewnetrznym.
W kazdym opakowaniu znajduja. si§ dwa worki i ulotka informacyjna.
PODMIOT ODPOWIEDZIALNY I WYTWORCA:
Gambro Lunriia AB Magistratsvagen 16 SE-220 10 Lund SZWECJA
PRODUCENT:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO) WLOCHY
Data ostatniej aktualizaeji ulotki: 07/2012
The following information is intended for healthcare professionals only
Prismasol® 2 mmol/l Potassium Solution for haemodialysis/ haemofiltration
PRECAUTIONS:
Carefully follow the instructions for use/handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is dear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock.
METHOD OF ADMINISTRATION:
Intravenous use and for haemodi-
alyais. Prismasol, when used as a
substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (postdilution).
POSOLOGY:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume Is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and
adolescents: 500 - 3000 ml/hour Children: 15-35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and
adolescents: 500 - 2500 ml/hour Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a dally amount of 55 i,
INSTRUCTIONS FOR HANDLING:
The solution Is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged.
All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no
longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/ or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions Is 7.0 to 8.5). Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
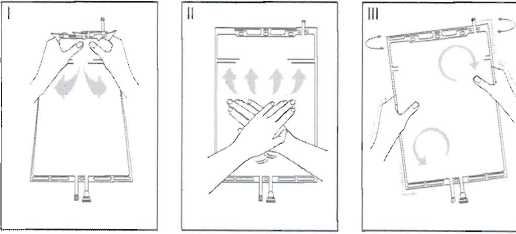
i Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
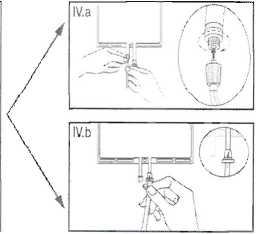
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag: tighten. Using thumb and fingers, break the blue frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure IV.a below) IV.taIf the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-renal replacement equipment.
STORAGE PRECAUTIONS:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If net used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
Pnsmd&cl 2 DG5725QG1 Rev 2013-02 4712
Azalabbi informaciok kizarolag egeszsegiigyi szakembereknek szolnak
Prismasol* 2 mmol/l kalium Hemodializaio vagy hemofiltracios oldat
OVINTEZKEDESEK:
Gondosan kdvesse a hasznalati es kezelesi utasitast.
Felhasznalas elott az elektrolitoldatot ossza kail keverni a pufferoldattal. hogy letrejojjon az aikalmazasra kesz oldat, amely alkalmas a hemofiltraciora, a hemodiafiltraciora vagy a folyama-tos hemodializisre.
Az oldat testhomersekletre (+37°C) vald felmelegitesekor gondosan ellenorizni kell, hogy az oldat tiszta-saga megorzodott-e szemcsekep-zodes nelkul,
Akallumszintet szorosan nyomon kell kovetni a legmegfelelobb kali-umkoncentracio kivalasztasahoz.
A szervetlen foszfat koncentra-ciojat rendszeresen merni kell, A szervetlen foszfatot potolni kell olyan esetekben, amikor a verben alacsony a foszfatszint.
A folyadekegyensuly zavarai eseten a klinikai allapotot gondosan nyomon kell kovetni, es a folyadek-egyensulyt helyre kell allitani. Szennyezett hemofiltracios es hemodializaio oldat alkalmazasa vermergezest es sokkot okozhat.
ALKALMAZAS MODJA:
Intravenas alkalmaz.asra es
hemodializishez. Potio oldatkent a Prismasolt beadhatjak a korbe a hemofiltraclo elott (predilucio) vagy utan (posztdilucio).
ADAGOLAS:
A Prismaso! alkalmazando mennyi-sege a beteg klinikai allapotatol es az elerni kivant folyadekegyensuly-toi fugg, Az alkalmazando meny-nyiseget ezert a kezelesert felelds orvos megltelese hatarozza meg.
A pdtlo oldat lehetseges aramlasi sebessegei hemofiltraclo es hemodiafiltracio eseten az alabbiak:
Felnotteknel es
serdiiloknei: 500 - 3000 ml/ora
Gyermekeknel: 15-35 ml/ttkg/ora Adializald oldat (dializatum) lehetseges aramlasi sebessegei folyamatos hemodializis es folya-matos hemodiafiltracio eseten az alabbiak:
Felnotteknel es
serdiiloknei: 500 - 2500 ml/6ra
Gyermekeknel: 15-30 ml/ttkg/ora Felnotteknel altaiaban a 2000 ml/ora koruli aramlasi sebes-seget hasznaljak, ami megkdzeli-toleg napi 55 Uteres mennyisegnek felel meg,
KEZELESI UTASITAS:
P-j. oldat ketrekeszes zsak kiszere-lesben kerul forgalomba. a betegnek valo adaaolas soran vegig aszeptikus modon kell eljarni, Kizarolag akkor hasznalja fel. ha az oldat tiszta, valamint sertetlen
a kulso csomagolas. Minden leza-
rasnak sertetlennek kell lenme. Ha szivargast eszlel. azonnal dobja ki az oldatot, mivel a sterllitas tobbe mar nem garantalhato.
A nagyobbik (B) rekeszhez egy injekcios bemenet tartozik, szuk-seg eseten ebbe lehet adagolni tovabbi gyogyszereket az oldat osszeallitasa utan. A kezeloorvos felelossege annak megltelese, hogy a Prismasol oldattal egyutt beadott gyogyszer kompatibilis-e az oldattal; emellett ellenoriznie kell a szinvaltozast es/vagy a kicsapd-das, illetve oldhatatlan komplexek es kristalyok kepzodesenek jeleit. Nezze at a hozzaadnl kivant gyogy-szer Alkalmazasi eloirasat,
Egyeb gyogyszer hozzaadasa elott ellenorizze, hogy az a Prismasol oldat pH-ertekenek megfelelo vizes kdzegben oldhato es stabil-e (a fel-hasznalasra kesz oldat pH-erteke 7.0-8,5).
Az oldathoz kizarolag a kezeloorvos felelossegere lehet tovabbi gyogyszereket adni, a kovetkezo modon: Tavotitson et minden folya-dekot az injekcios bemenetbol, tart-sa fejjel lefele a zsakot, viaye be a gyogyszer! az injekcios bemeneten keresztul, majd alaposan keverje ossze az oldatot. A kesz oldatot azonnal be kell adni.
i Kozvetlenul a felhasznalas elott vegye le a zsakrol a kulso csomagolast, 6s keverje ossze a ket rekesz tartatmat. A kiseb-bik rekeszt mindket kezevel tartva preselje addig. amig meg nem nyilik benne az atjaras a ket rekesz kozott. (Lasd az alabbi I. abrat.)
II Nyomja ossze a nagyobbik rekeszt mindket kezevel addig. amig teljesen meg nem nyilik a lezaras a ket rekesz kdzStt. (Lasd az alabbi II. abrat.)
III Ugyeljen az oldat bizios ossze-keveredesere, razza fel ovato-san a zsakot. Az oldat most mar keszen 611 a felhasznalas-ra, es felakaszthatja a keszti-lekre. (Lasd az alabbi III. abrat.)
IV A dializald folyadek vezeteke vagy a potlo oldat vezeteke a ket bemeneti nyilas barmelyi-Kehez csatlakoztathato.
IV.a Ha a Luer-csatlakozos beme ■ netet hasznalja, vegye le a ku-pakot, csatlakoztassa a dializald vagy a potlo oldat ve-zetekenek apavegu Luer-zarjat a zsakon levd anyavegu Luer-aljzatba. majd szoritsa meg. Huvelykujja es ujjai segitsege-vel tdrje el a kek, konnyen el-torheto csatiakozocsapot a to-venel, majd huzza vissza es elore. Ne hasznaljon ehhez se-gedeszkdzt. Ellenorizze, hogy a csatlakozocsap teljesen szet-valt-e. es hogy a folyadek sza-badon tud-e aramlani. A csatlakozocsap a kezeles iaeje alatt a Luer-nyilasban marad. (Lasd az alabbi IV.a abrat.)
IV.bHa az injekcios bemenetet hasznalja. eldszor vegye le a vedosapkat. Ezutan szurja at a tiisket a gumimembranon. Ellenorizze, hogy a folyadek sza-badon aramlik-e. (Lasd az alabbi IV.b. abrat)
KULONLEGES TAROLASI ELOiRASOK:
Az alkalmazasra elokeszitett oldat hasznalat kozbeni kemiai es fizikai stabilitasat 24 oran at vizsgaltak +22''C-on. Kemiai tenyezok miatt az osszeallitott oldatot azonnal fel kell hasznalni. Ha a kesz oldatot nem hasznaljak fel azonnal. az oldat tarolasi idejeert es korulme-nyeiert a felhasznalo felelos; a kesz oldat tarolasa ne tartson tovabb 24 oranal, beleertve a kezelds iaotartamat is
A kesz oldat kizarolag egyszeri felhasznalasra szolgal. A fel nem hasznalt oldatot az alkalmazas befejezese utan azonnal dobja el.
Kizarolag megfeleio extrarenalis vesepotlo berendezessel hasznal-hato.
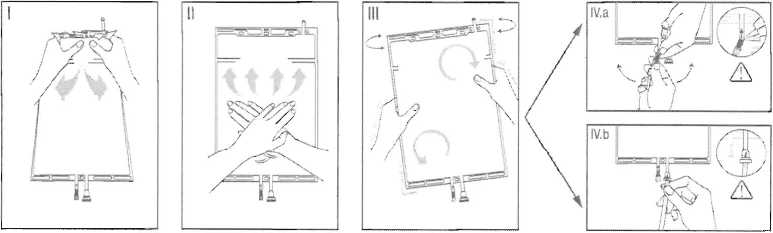
Informacje przeznaczone wylqcznie dla fachowego personelu medycznego
Prismasol* 2 mmol/l potasu Roztwor do hemodiaiizy/ hemofittracji
SRODKI OSTROZNOSCI:
Doktadnie przestrzegac instrukcji uZycia/post^powania.
Roztwor elektrolitow musi zostac zmleszany z roztworem bufo-rowym przed uzyciem w celu uzyskania koncowego roztworu do hemofittracji/hemodiafiitracji lub cigglej hemodiaiizy.
Nalezy starannie kontrolowac ogrzewanie roztworu do temperatu-ry data (+37°C), sprawdzaj^c, czy roztwor jest przezroczysty i wolny od cz^stek staiych.
Nalezy doktadnie monitorowac kaliemie w celu umozliwienia prawidtowego wyboru najodpowied-niejszego stezenia potasu.
Nalezy regularnie mierzyc stezenie nieorganicznych fosforandw. W przypadku hipofosfatemii (niskie-go stezenia fosforandw we krwi). nieorganiczne fosforany nalezy uzupetniac
W przypadku braku rownowagi ply-now nalezy starannie monitorowac stan kiiniczny pacjenta i przywracac normalny bilans ptynow,
Uzycie zanieczyszczonych roztwo-row do hemofiltracji i hemodiaiizy moze spowodowac posocznice i wstrz^s.
SPOSOB PODAWANIA:
Do podavvania dozylnego i hemodiaiizy. Prismasol 2 mmol/l potasu uzywany jako roztwor zamienny jest podawany do obwodu przed hemofiltrem (predylucja) lub za hemofiltrem (postdylucja).
DAWKOWANIE:
Objetosc uzywanego roztworu Prismasol 2 mmol/l potasu zaiezy od stanu klinlcznego pacjenta i docelowego bilansu ptynow. Dlatego decyzje o objetosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Szybkosci przeptywu dla roztworu zamiennego w hemofiltracji i hemo-ciiafiitracji wynosza:
Dorosli i
mfodziez: 500 do 3000 ml/godz
Dzieci: 15 do 35 ml/kg mc./godz.
Szybkosci przeptywu dla roztworu do dializy (dializaf) w ciegtej he-modializie i ciggfej hemodiafiltracji wynosz^:
Dorosli i
mtodziez: 500 do 2500 ml/godz.
Dzieci: 15 do 30 ml/kg mc./godz.
Najczesciej stosovvane szybkosci przeptywu w przypadku dorostych wynosza okoto 2000 ml/godz.. co odpowiada dziennej objetosci wynoszgcej 55 i.
INSTRUKCJA POST^POWANIA:
Roztwor jest pakowany w dwuko-morowe worki.
Podczas podawania leku pacjen-towi nalezy stosowactechnike aseptyczna_.
Uzywac wyt^cznie wtedy, gdy roztwor jest przezroczysty, a zewn^li zne opakowanie nieusz-
kodzone. Wszystkie spawy musza bye nienaruszone. W przypadku zauwazania przecieku roztwor nalezy niezwlocznie wyrzucic, po-niewaz nie moZna zagwarantowac jalowosci.
Duza komora B wyposazona jest w port do wstrzykniec, umozliwiajacy dodanie po odtworzeniu/zmiesza-niu do roztworu innych koniecznych lekow. Lekarz pozostaje odpowiedzialny za okreslenie niezgodnosci lekow dodatkowych z roztworem Prismasol za pornoca sprawdzania zmiany koloru i (lub) wytwarza-nia osadu, nierozpuszczalnych kompleksow lub krysztatdw. Nalezy zapoznac sie z instrukcji uZycia dodawanego leku.
Przed dodaniem leku nalezy sprawdzic, czy jest rozpuszczalny i stabilny w wodzie o pH takim jak roztwor Prismasol 2 mmol/l potasu (pH odtworzonego/zmieszanego roztworu wynosi od 7:0 do 8,5).
Leki powinny bye dodawane do roztworu pod kontroli lekarza w nastepujacy sposob: Usunac ptyn z portu do wstrzykniec, przytrzymac worek do gory nogami, wstrzykna_c lek przez port i starannie wymie-szac. Roztwor rtalezy podac niezwtocznie.
i Bezposrednio przez uzyciem zdjac zewnetrzne opakowanis z vvorka i wymieszac roztwory z dwoch roznych komor. Uchwy-cic mai^ komor? dtorimi i sci-sn?c do momentu powstania otworu vv rczrywalnyrn spawie oddzielajacym obydwie komo-ry. (Patrz rysunek I ponizej).
II Obiema dlonmi nacisnac duza komor? do momentu catkowiie-go otwarcia rozrywalnego spa-wu pomi?dzy dvvofna komora-mi, (Patrz rysunek II ponizej).
III Catkowicie wymieszac roztwor, wstrza.sajac delikatnie worek. Roztwor jest teraz gotovvy do uzycia, a worek mozna zawie-sic na stojaku. (Patrz rysunek III ponizej)
fV Do kazdego z dwoch portow dostepu mozna podl^czyc lini? dializy lub wymiany.
IV.aWprzypadku korzystania ze ztacza typu Luer nalezy zdjgc osionk? i podl^czyc m?ska koncowk? typu Luer linii dializy lub wymiany do zehskiego l^cznika Luer w worku. dokr?-cic. Chwytajqc kciukiem i pal-cami odiamac niebiesk^ lamli-vva zatyczk? u podstawy i poru-szac nia tarn i z powrotem. Nie nalezy uzywac narzedzia. Nalezy upewnic si?, ze zatyczka oddzielita si? catkowicie i ze plyn moze przep)ywac swobod-nie. Zatyczka podczas terapi pozostaje w porcie Luer. (Patrz rysunek IV.a ponizej).
IV.bW przypadku korzystania z portu do wstrzykni?c najpierw nalezy zdj^c kapsel. Nast?pnie wprowadzic kolec przez gumo-wa przegrod?. Sprawdzic, czy ptyn przeptywa swobodnie. (Patrz rysunek IV.b ponizej).
SRODKI OSTROZNOSCI PRZY PRZECHOWYWANiU:
Wykazano chemiczn^ i fizycznq stabilnosc odtworzonego roztworu w ciagu 24 godzin w temperaturze +22'C. Z chemicznego punktu widzenia odtworzony roztwor powinien zostac niezwlocznie uzyty. Jesli nie zostanie zuzyty od razu, za czas i warunki przechowy-wania przed uzyciem odpowiada uzytkownik i normalnie czas ten nie powinien przekraczac 24 godzin iacznie z czasem zabiegu, Odtworzony roztwor nie nadaje sie do powtornego uzycia. Usunac caiy niezuzyty roztwor bezposrednio po uzyciu.
Nalezy uzywac wyt^cznie z odpow-iednimi urzadzeniami do wymiany pozanerkowej.
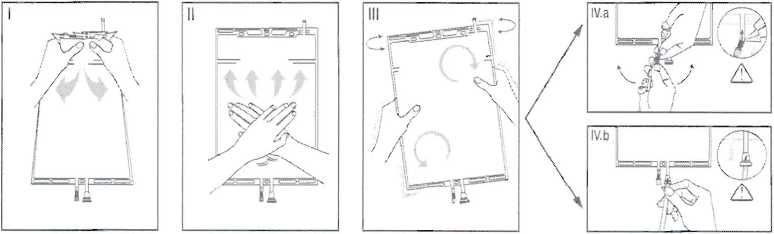
Prismasol arid Gambro are trademarks belonging to the Gambro Group
Gambro Lundia AB P 0 Box 10101 SE-220 1 0 Lund Sweden
Visiting address. Magistralsvagen 16. Lund Tel +46 46 16 SO 00 www. g a m oro. com
^GAMBRO,
Package leaflet: information for the user
Prismasol 2 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 2 mmol/l Potassium is indicated particularly in patients who have tendency to hyperkalaemia (a high concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 2 mmol/l Potassium in the following cases:
• Hypokalaemia (a low concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
other . Your
Tell your doctor or pharmacist if you are given, have recently been given or might be given any medicines.
The blood concentration of some of your other medicines may be reduced during the treatment doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.157 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
111.50 |
111.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
hco3- |
32.00 |
32.00 |
|
Potassium |
K+ |
2.00 |
2.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
297 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the frangible pin and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only:
Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two compartment-bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Remove the overwrap from the bag immediately before use and discard any other packaging materials. Open the seal by breaking the frangible pin between the two compartments of the bag. The frangible pin will remain in the bag. (See figure I below)
II Make sure all the fluid from the small compartment A is transferred into the large compartment B. (See figure II below)
III Rinse the small compartment A twice by pressing the mixed solution back into the small compartment A and then back into the large compartment B. (See figure III below)
IV When the small compartment A is empty: shake the large compartment B so that the contents mix completely. The solution is now ready for use and the bag can be hung on the equipment. (See figure IV below)
V The dialysis or replacement line may be connected to either of the two access ports.
V.a If the luer access is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure V.a below)
When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
V.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure V.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22° C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
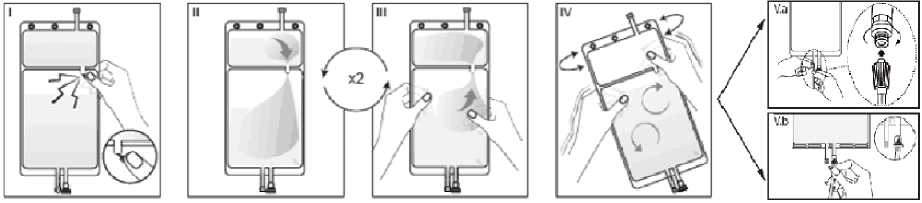
Package leaflet: information for the user
Prismasol 2 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 2 mmol/l Potassium is indicated particularly in patients who have tendency to hyperkalaemia (a high concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 2 mmol/l Potassium in the following cases:
• Hypokalaemia (a low concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
other . Your
Tell your doctor or pharmacist if you are given, have recently been given or might be given any medicines.
The blood concentration of some of your other medicines may be reduced during the treatment doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
Possible side effects
4.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium hydrogen carbonate 3.090 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
111.50 |
111.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
hco3- |
32.00 |
32.00 |
|
Potassium |
K+ |
2.00 |
2.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
297 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the frangible pin and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE-220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only:
Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Remove the overwrap from the bag immediately before use and discard any other packaging materials. Open the seal by breaking the frangible pin between the two compartments of the bag. The frangible pin will remain in the bag. (See figure I below)
II Make sure all the fluid from the small compartment A is transferred into the large compartment B. (See figure II below)
III Rinse the small compartment A twice by pressing the mixed solution back into the small compartment A and then back into the large compartment B. (See figure III below)
IV When the small compartment A is empty: shake the large compartment B so that the contents mix completely. The solution is now ready for use and the bag can be hung on the equipment. (See figure IV below)
V The dialysis or replacement line may be connected to either of the two access ports.
V.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or
replacement line to the female luer receptor on the bag: tighten. Using thumb and fingers, break the blue frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure V.a below)
V.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure V.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22° C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
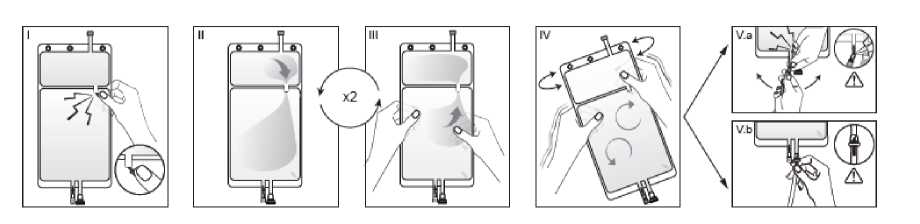
Package leaflet: information for the user
Prismasol 2 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/
lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen
carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 2 mmol/l Potassium is indicated particularly in patients who have tendency to hyperkalaemia (a high concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 2 mmol/l Potassium in the following cases:
• Hypokalaemia (a low concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for
haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.157 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
111.50 |
111.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
hco3- |
32.00 |
32.00 |
|
Potassium |
K+ |
2.00 |
2.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
297 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only: Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock.
Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure IV.a below).
When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
IV.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24
hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
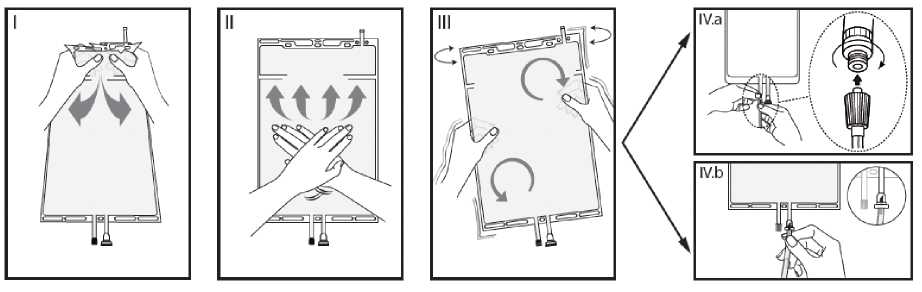
Package leaflet: information for the user
Prismasol 2 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/
lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen
carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 2 mmol/l Potassium is indicated particularly in patients who have tendency to hyperkalaemia (a high concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 2 mmol/l Potassium in the following cases:
• Hypokalaemia (a low concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor , pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
Possible side effects
4.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium hydrogen carbonate 3.090 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
111.50 |
111.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
HCO3" |
32.00 |
32.00 |
|
Potassium |
K+ |
2.00 |
2.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
297 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections
pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only: Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag; tighten. Using thumb and fingers, break the blue frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure IV.a below)
IV.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.

Prismasol 2 / UK / PL polyolefin luer connector with pin / 2012-07 6/6
DO NOT USE PRISMASOL 2 MMOL/L POTASSIUM IN THE FOLLOWING CASES:
• Hypokalaemia (a low concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
DO NOT USE
HAEMOFILTRATION/ DIALYSIS IN THE FOLLOWING CASES:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
WARNINGS AND PRECAUTIONS
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
NEALKALMAZZAA PRISMASOL 2 MMOL/L KALIUM KESZITMENYT AZ ALABBI ESETEKBEN;
« Korosan alacsony kali-umkoncentracio a verben (hipokalemia)
• A ver vegyhatasanak lugos iranyu eltolodasa (metabolikus alkalozis, a plazma bikarbonat-koncentracidjanak megemel-kedeseert elsGdlegesen feielos folyamat)
NEM VEGEZHETQ HEMOFILTRACIO/DIALiZIS A KOVETKEZO ESETEKBEN:
• Veseeiegtelenseg hangsulyos hiperkatabolizmussal (kdro-san megndvekedett lebomlasi folyamatok a szervezetben), ha a hemofiltracio nem kepes a karbamidnak a verben vald magas koncentracioja altal okozott tunetek (az uremias tunetek) elharitasara.
» Elegtelen arterias nyornas a vererekhez vald hozzaferas szempontjabol.
Csokkent veralvadasi kepesseg (szisztemas antikoagulalas), ha nagy a verzes kockazata.
FIGYELMEZTETESEK ES OVINTEZKEDESEK
A Prismasol alkalmazasa elStt be-szeljen kezeloorvosaval, gyogysze-reszevel vagy a szakszemelyzettel. Az oldat kizardlag a veseeiegtelenseg hemofiltracioval. hemodiafiltracioval es folyamatos
NIE NALEZY
STOSOWAC ROZTWORU PRISMASOL 2 MMOL/L POTASU. JESLI:
• u pacjenta wystepuie hipoka-liemia (niskie stpzenie potasu we krwi),
• u pacjenta wystepuje alkaloza metaboliczna (proces, ktory po-woduje gtownie wzrost stezenia wodoroweglanu w osoczu).
NIE STOSOWAC HEMOFILTRACJI/DIALIZY W NASTEPUJACYCH PRZYPADKACH:
• niewydolnosc nerek z wyraznym hiperkatabolizmem (niepra-widlowy wzrost katabolizmu), jesli objawow uremii (objawy spowodowane wysokim stg-zeniem mocznika we krwi) nie mozna zlagodzic za pomoca hemofiltracji;
• niewystarczajace cisnienie t?tni-cze w dosteple naczyniowym;
antykoagulacja ogolnoustrojowa (zmniejszona krzepiiwosc krwi) przy zagrozeniu krwotokiem (krwawleniem),
OSTRZEZENIA I SRODKI OSTROZNOSCI
Przed rozpocz^ciem przyjmowanla roztworu Prismasol nalezy zwrocic slg do lekarza, farmaceuty lub pielegniarki.
DO NOT USE PRISMASOL 2 MMOL/L POTASSIUM IN THE FOLLOWING CASES:
• Hypokalaemia (a low concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
DO NOT USE
HAEMOFILTRATION/ DIALYSIS IN THE FOLLOWING CASES:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
WARNINGS AND PRECAUTIONS
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g, your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
NEALKALMAZZA A PRISMASOL 2 MMOL/L KALIUM KESZITMENYT AZ ALABBI ESETEKBEN:
• Korcsan alacsony kali-umkoncentracio a verben (hipokalemia)
• A ver vegyhatasanak lugos iranyti eltolodasa (metabolikus alkalozis, a plazrna bikarbonat-koncentraciojanak megemei-kedeseert elsodlegesen felelos foiyamat)
NEM VEGEZHETO HEMOFILTRACIQ/DIALIZIS A KOVETKEZO ESETEKBEN:
B Veseelegtelenseg hangsulyos hiperkatabolizmussal (koro-san megnovekedett lebomlasi folyamatok a szervezetben), ha a hemofiltracio nem kepes a karbamidnak a verben valo magas koncentracioja altal okozott tunetek (az uremias tunetek) elharitasara,
• Elegteien arterias nyomas a vererekhez valo hozzaferes szempontjabol.
Csokkent veralvadasi kepesseg (szisztemas antikoaguldlas) ha nagy a verzes kockazata,
FIGYELMEZTETESEK ES OVINTEZKEDESEK
A Prismasol alkalmazasa elott be-szeljen kezeloorvosaval, gydgysze-reszevel vagy a szakszemelyzettel. Az oldat kizarolag a veseelegtelenseg hemofiltracioval, hemodiafiltracioval es folyamatos
NIE NALEZY
STOSOWAC ROZTWORU PRISMASOL 2 MMOL/L POTASU, JESLI:
• u pacjenta wystppuje hipoka-liemia (niskie stpzenie potasu we krwi),
• u pacjenta wyst?puje alkaloza metaboliczna (proces, ktory po-woduje gfownie wzrost stpzenia wodoroweglanu w osoczu),
NIE STOSOWAC HEMOFILTRACJI/DIALIZY W NASTEPUJACYCH PRZYPADKACH:
• niewydolnosd nerek z wyraznym hlperkatabolizmem (niepra-widiowy wzrost katabolizmu), jesli objawow uremii (objawy spowodovvane wysokim ste-zeniem mocznika we krwi) nie mozna ztagodzic za pornoc? hemofiltracji;
• niewystarczajpce cisnienie tptni-cze w dostppie naczynidwym;
antykoagulacja ogolnoustrojowa (zmniejszona krzepliwosc krwi) przy zagrozeniu krwotokiem (krwawieniem).
OSTRZEZENIA I SRODKI OSTROZNOSCI
Przed rozpoczeciem przyjmowania roztworu Prismasol nalezy zwrocic si? do lekarza. farmaceuty lub pielegniarki.