Prismasol 4 Mmol/L Potassium Solution For Haemodialysis/Haemofiltration
Prismasol® 4
UK, IE, MT Package leaflet: Information for the user.............................3
PL Uiotka dla pacjenta..............................................................5
X.................................- - X
UK, IE, MT The following information is intended
for healthcare professionals only.........................................7
PL Informacje przeznaczone wyt^cznie dla
fachowego personelu medycznego.....................................9
"L.sj 43. -tj-ot
**GAMBRO
THIS PAGE IS INTENTIONALLY LEFT BLANK
PrismasoF 4 mmol/l Potassium Solution for haemodialysis/ haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains Important information for you.
• Keep this leaflet. You may need
• If you have any further questions, ask your doctor, pharma-
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet,
WHAT IS IN THIS LEAFLET:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. WHAT PRISMASOL IS AND WHAT IT IS USED FOR
Prismasol contains the active substances calcium chloride dlhydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w. sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltra-tion (as a replacement for fluid lost from the blood passing through a Alter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasolsolution may also be
used in case of drug poisoning with dialysable or filterable substances. Prismasol 4 mmol/l Potassium is indicated particularly in patients who are normokalaemic (a normal concentration of potassium in the blood). 1
OTHER MEDICINES AND PRISMASOL
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis Is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hyper-calcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
PREGNANCY AND BREASTFEEDING
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. As all medicines, your doctor will decide If you should be given Prismasol if you are pregnant or breast-feeding.
DRIVING AND USING MACHINES
Prismasol is not known to affect your ability to drive or use ma-
3. HOW PRISMASOL IS USED
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor. Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
IF YOU THINK YOU ARE GIVEN MORE PRISMASOL THAN YOU THINK YOU SHOULD BE
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result In fluid overload if you suffer from renal failure. Overdose could lead to severe consequences, such as congestive heart failure, electrolyte cr acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration. the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or
4. POSSIBLE SIDE EFFECTS
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. HOW TO STORE
PRISMASOL_
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
WHAT PRISMASOL CONTAINS The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
1000 ml of buffer solution (from the large compartment (B)) contains Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
.................... inmol/l mEg/l
Calcium, CtF.................... " 1.75............3.50
Sodium, Na' 140.00 140.00
Chloride, Cl 113.50 113.50
Hydrogen
Theoretical Osmolarity:
301 mOsnn/l
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution:
7.0-8.5
WHAT PRISMASOL LOOKS LIKE AND CONTENTS OF THE PACK
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B. the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
MARKETING AUTHORISATION HOLDER
Gambro Lundia AB Magistratsvagen 16 SE-220 10 Lund SWEDEN MANUFACTURER Gambro Dasco S.p.A.
Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012
Prismasol' 4 mmol/l potasu Roztwor do hemodializy/ hemofiltracjl
Wapnia chlorek dwuwodny/ magne-zu chlorek szesdowodny/ glukoza jednowodna/ kwasu mlekowego roztwor 90% w/w / sodu chlorek/ potasu chlorek/ sodu wodorowe-glan
Nalezy zapoznac sie z trescia ulotkl przed przyjeciem leku, poniewaZ zawiera ona Informacje wazne dla pacjenta.
• Nalezy zachowac te ulotk?, aby w razie potrzeby moc ja ponow-nie przeczytac.
• NaleZyzwrdcid sie do lekarza. farmaceuty lub pielegniarki w razie jakichkolwiek dalszych watpliwodcl.
• Jedli nasili sie ktorykolwiek z objawow niepozadanych lub wystapia jakiekolwiek objavvy niepozgdane. w tym niewy-mienione w ulotce, nalezy powiedzied o tym lekarzowi. farmaceucie lub pieiegniarce.
SPIS TRESCI ULOTKI:
1. Co to jest Prismasol 4 mmol/l potasu I w jakim celu sie go stosuje
2. Informacje wazne przed przyjeciem roztworu Prismasol 4 mmol/l potasu
3. Jakstosowac Phsmasol 4 mmol/l potasu
4. Mozliwe dzialania mepozadane
5. Jak przechowywac Prismasol 4 mmol/l potasu
6. Zawarlosd opakowania i inne Informacje 2
krwi przeplywajacej przez filtr) oraz ciEtgtej hemodializy lub hemodiafiltracji (krew przeptywa po jednej stronie membrany dializacyjnej. podczas gdy raztwdr do hemodializy przeptywa po drugiej stronie membrany).
Prismasol 4 mmol/l potasu moZna rownleZ stosowadw przypadku zatrucia lekami zawierajacymi substancje ulegajace dializie lub filtracji.
Prismasol 4 mmol/l potasu jest wskazany u pacjentow, majacych normalne st?2enie potasu we krwi.
2. INFORMACJE WA2NE PRZED PRZYJECIEM ROZTWORU PRISMASOL 4 MMOL/L 3 4
rem lekarza wykwalifikowanego w leczeniu niewydolnosci nerek przez zastosowanie hemofiltracji, hemodiafiltracji i ciaglej hemodializy. Przed zastosowaniem leczenia i podczas niego zostanie zbadana krew pacjenta, np. rdwnowaga kwasowo-zasadowa i st^zenie elektrolitbw (soli we krwi).
NaleZy dokladnie monitorowac ste-Zenie glukozy we krwi, zwtaszcza gdy paejent choruje na cukrzyc§. INNE LEKII
PRISMASOL 4 MMOUL POTASU
NaleZy powiedzied lekarzowi lub farmaceucie o wszystkich lekach przyjmowanych obecnie lub ostat-nio a takze o lekach, ktore paejeni planuje przyjmowad.
Stezenie we krwi innych przyjmowanych lekdw moZe zostac obnizone podczas leczenia. Lekarz zdecyduje, czy wymagana jest zmiana przyjmowanych lekow.
W szczegolnosci naleZy powiedzied lekarzowi o przyjmowaniu nastepu-jticych lekow:
• glikozydow (stosowanych w leczeniu okreslonych chorob serca), gdyZ podczas hipokalie-mii (obniZonego steZenia potasu we krwi) zwipkszaja one ryzyko wystapienia zaburzeh rytmu serca (nieregularnego lub przy-spieszonego Bicia serca):
• wltaminy U i produktow lecz-niezyeh zawierajacych wapri, gdyZ moga one zwi^kszac ryzyko wystapienia hiperkalce-mii (wysokiego st?zenia wapnia we krwi);
• jakichkolwiek dodatkdw wodo-roweglanu sodu obecnych w innych lekach, gdyZ moZe on zwiekszac ryzyko wystapienia zasadowicy metabolieznej (nadmiaru wodoroweglanow
ClAiA I KARMIENIE PIERSIA
W ciaZy i w okresle karmienla pier-sia lub gdy istnieje podejrzenie. ze kobieta jest w ciaZy. lub gdy planuje ciaZp, przed zastosowaniem tego leku nalezy poradzic si? lekarza lub farmaceuty.
Tak jak w przypadku wszystkich lekow. lekarz zadecyduje o poda-waniu roztworu Prismasol kobietom w ciaZy lub karmlacym piersla.
315/2013 17:46513
i-PVC UK It MT PI luer »ilh valve 2013-03.mdd
1000 ml roztworu eiektrolitowego (mala komora A) zawiera:
Wapnia chlorek dwuwodny 5.145 g
Po odtworzeniu/zmieszaniu: Roztwory w komorach A (250 ml)
I B (4750 ml) sp mieszane w celu otrzymania jednego odtworzonego/ zmieszanego roztworu (5000 ml) skladajpcego si? z:
8oo'
PROWADZENIE POJAZD6W I OBSLUGA MASZYN
Nie wiadomo, czy Prismasol wplywa na zdolnosc prowadzenia pojazdow i obstugi maszyn.
3. JAK STOSOWAC PRISMASOL 4 MMOUL
POTASU_
Ten lek nalezy zavvsze stosowad zgodnie z zalecenlami lekarza.
W razie wptpliwodci nalezy zwrocic sie do lekarza lub farmaceuty. Gbj?tosc uZywanego roztworu Prismasol 4 mmol/l potasu zalezy od stanu klinicznego pacjenta i oocelowego bilansu plynovv. Dlatego decyzj? o obj?tosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Droga podawania: do podawania doZylnego i hemodializy.
Instrukcje uZycia znajduja. sip w czesci JnformacJe przeznaczone wytpcznie dla fachowego personelu medycznego".
GDY PACJENTOWI WYDAJE SIE, ZE PODANO MU WIEKSZA ILOSC ROZTWORU PRISMASOL NIZ ZALECANA
Nalezy doktadnie monitorowac bilans plyndw, rownowag? elektroli-tewa i kwasowo-zasadowp. Przedavvkowanie mode spowodo-wac przecipzenie plynami u pacjen-tdw z niewydolnoscia nerek. Przedavvkowanie moze prowadzic do powaZnych nastppstw, takich jak zastoinowa niewydolnascserca, zaburzenia rownowagi elektrolito-vvej czy kwasowo-zasadowej.
Ciagte stosowanie hemofiltracji usuwa nadmiar plynu i elektrolitow. W przypadku przewodnienia nalezy zwipkszyc ultrafiltracj? i zmniejszyc wielkodd przeplywu roztworu do hemofiltracji. W przypadku ci?z-kiego odwodnienia konieczne jest przerwanie ultraflltracji i odpowied-nie zwipkszenie naplywu roztworu do hemofiltracji.
W razie jakichkolwiek dalszych watpliwosci zwipzanych ze stoso-waniem tego leku nalezy zwrocic si? do lekarza, farmaceuty lub pielegniarki.
Moziiwe sp nastppuja.ee dziatania niepozadane zwia.zane z uzywa-niem roztworu: przewodnienie lub odwodnienie (nieprawidfowo wysoka lub niska objetosc wody w organizmie), zaburzenia elektro-litowe (sole mineraine we krvvi), hipofosfatemia (nieprawidtowo niskie stpzenie fosforanow we krwi), hiperglikemia (nieprawidlowo wysokie stpzenie glukozy we krwi) i alkaloza metaboliczna (proces, ktdry powoduje gtownie zwipksze-nie stpzenia wodorowpglanu w osoczu).
Mogp wystapic pewne reakcje niepozadane zwipzane z dializa, np. nudnodci, wymioty, skurcze miesni oraz niedocisnienie (niskie cisnienie krwi).
Jesli wystapip jakiekolwiek objawy niepozadane, w tym wszelkie moziiwe objawy niepozadane niewymienione w ulotce, naleZy zwrdcld si? do lekarza, farmaceuty lub pielegniarki.
5. JAK PRZECHOWYWAC PRISMASOL 4 MMOUL POTASU_
Lek nalezy przechowywac w miej-scu niewidocznym i niedost?pnym dla dzieci.
Nie przechowywac w temperaturze poniZej +4°C.
Nie stosowac tego leku po uplywie terminu waZnodci zamieszczonego na etykiecie i opakowaniu. Termin waznosci oznacza ostatni dziert danego miesiaca.
6. ZAWARTOSC OPAKOWANIA I INNE INFORMACJE
CO ZAWIERA
PRISMASOL 4 MMOUL POTASU Substancje czynne:
Przed odtworzeniem/zmiesza-
Magnezu chlorek
Glukoza bezwodna 22,000 g
1000 ml roztworu buforowego (duia komora B) zawiera:
Sodu wodorowpglan 3.090 g
_ mmol/l mEq/l
Chlorki, Clp 113,50 113,50
Wodorowpglan, HCO; 32.00 32,00
Teoretyczna osmolamosc:
301 mOsm/l
Inne skfadniki leku to: dwutlenek wegla, woda do wstrzykiwari. pH odtworzonego/zmieszanego roztworu wynosi: 7.0 do 8.5
JAK WYGLADA
PRISMASOL 4 MMOL/L POTASU I CO ZAWIERA OPAKOWANIE
Prismasol 4 mmol/l potasu jest pakowany w dwukomorowe worki zawierajpce w mniejszej komorze A roztwor elektrolitow, a w wipkszej komorze B - roztwor buforowy. Oslateczny odtworzony/zmieszany roztwor uzyskuje sie po rozerwa-niu spawu i wymieszania obu roztworow. Odtworzony/zmieszany roztwor jest przezroczysty i lekko zdlty. Kazdy worek (A+B) zawiera 5000 ml roztworu do hemofiltracji i hemodializy. Kazdy worek umieszczonyjestw przezroczystym opakowaniu zewnptrznym.
W kazdym opakowaniu znajduja si? dwa worki i ulotka informacyjna. PODMIOT ODPOWIEDZIALNY I WYTWORCA Gambrp Lundia AB Magistratsvagen 16 SE-220 10 Lund SZWECJA
PRODUCENT Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO) WtOCHY
07/2012
VC UK IE MT1
The following information is intended for healthcare professionals only
UK I IE i MT
Prismasol14 mmol/l Potassium Solution for haemodialysis/ haemofiltration PRECAUTIONS
Carefully follow the instructions for use/handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37'C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. METHOD OF ADMINISTRATION Intravenous use and for haemodialysis. Prismasol. when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
POSOLOGY
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and
adolescents: 500 - 3000 ml/hour Children: 15-35ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and
adolescents: 500 - 2500 ml/hour Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 551. INSTRUCTIONS FOR HANDLING The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is dear and the overwrap is undamaged.
All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/ or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5). Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
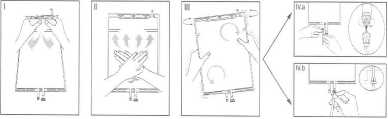
I Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the
compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, repull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure IV.a below).
When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable
IV.blf the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
STORAGE PRECAUTIONS
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use Storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
Use only with appropriate extra-renal replacement equipment.
Informacje przeznaczone wyt^cznie dla fachowego personelu medycznego
PL
Prismasol® 4 mmol/l potasu Roztw6r do hemodializy/ hemofiltracji SRODKI OSTROZNOSCI
Dokladnie przestrzegac instrukcji uiycia/post^powania.
Roztwir elektrolitow musi zostac zmleszany z roztworem bufo-rowym przed uzyciem wcelu uzyskania koricowego roztworu do hemofiltracji/hemodiafiliracji lub ciagiej hemodializy.
Nalezy starannie kontrolowac ogrzewanie roztworu do temperatu-ry data (+37°C), sprawdzaj^c. czy roztwor jest przezroczysty i wolny od cz^stek stalych.
Nalezy dokladme monitorowad kallemie w celu umozliwienia prawidtowego wyboru najodpowied-niejszego stpdenia potasu.
Nalezy regularnie mlerzyc steZenie nieorganicznych fosforanow. W przypadku hipofosfatemii (niskie-go stezenia fosforanow we krwi), nieorganiozne fosforany nalezy uzupelniad
W przypadku braku rownowagi ply-now nalezy starannie monitorowad stan kliniczny pacjenta i przyv/racab normalny bilans plynow.
Uzyeie zanieczyszczonych roztwo-rovv do hemofiltracji i hemodializy moze spowodowac posocznice i wstrzgs.
SPOSOB PODAWANIA
Do podawania doZylnego i hemodializy. Prismasol 4 mmol/l potasu uzywany jako roztwor zamienny jest podawany do obwodu przed (predylucja) lub za hemofiltrem (postdylucja),
DAWKOWANIE
Objetosc uzywanego roztworu Prismasol 4 mmol/l potasu zalezy od stanu klinicznego pacjenta i docelov/ego bilansu plynow. Dlatego decyzjp o objetosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Szybkosci przeplywu dla roztworu zamiennego w hemofiltracji i hemo-diafiltracji wynosza:
Dorosll i
mtodziez: 500 do 3000 ml/godz.
Dzieci: 15 do 35 ml/kg mc./godz, Szybkosci przeplywu dla roztworu do dializy (dializat) w cigglej he-modiaiizie i cigglej hemodiafiltracji wynoszq:
Dorosli I
mtodziez: 500 do 2500 ml/godz. Dzieci: 15 do 30 ml/kg mc./godz, Najczesciej stosowane szybkodcl przeplywu w przypadku doroslych wynosza okolo 2000 ml/godz.. co odpowiada dziennsj objetosci wynoszacej 55 I.
INSTRUKCJA POST^POWANIA
Roztwor jest pakowany w dwuko-morowe worki.
Podczas podawania leku pacjen-towi nalezy stosowac technikg aseptyczna..
Uzywac wylacznie wtedy, gdy roztwor jest przezroczysty. a zewnetrzna opakowanie nieusz-kodzone. Wszystkie spawy musza bye nienaruszone. W przypadku zauwazenia przecieku roztwor nalezy niezwlocznie wyrzucic, po-niewaz nie mozna zagwarantowac jalowosci.
Duza komora B wyposazona jesl w port do wstrzykiwart, umozliwiaja.cy dodanie po odtworzeniu/zmiesza-niu do roztworu innych koniecznych lekow. Lekarz pozostaje odpowiedzialny za okreslenie niezgodnosci lekow dodatkowych z roztworem Prismasol za pomoca sprawdzania zmiany barwy i (lub) wytwarza-nia osadu, nierozpuszczalnych kompleksbw lub krysztalow. Nalezy zapoznac sip z instrukcji uzyeia dodawanego leku.
Przed dodaniem leku nalezy sprawdzid, czy jest rozpuszczalny i stabilny w wodzie o pH takim jak roztwor Prismasol 4 mmol/l potasu (pH odtvvorzonego/zmieszanego roztworu wynosi od 7,0 do 8,5),
Leki powinny bye dodawane do roztworu pod kontrolej lekarza w nastepujicy spos6b: Usungd plyn z portu do wstrzykni^c, przytrzymac worek do g6ry nogami, wstizykn^c lek przez port i starannie wymie-szac. Roztwor nalezy podac niezwlocznie.
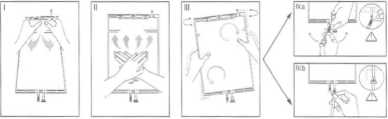
Bezposrednlo przez uzyciem zdjac zewnptrzne opakowanie z worka i wymieszac roztwory z dwoch rdznych komor. Uchwy-cic mala komorp dlorimi I sci-sna.c do momentu powstania otworu w rozrywalnym spawie oddzielajpcym obydwie komo-ry. (Patrz rysunek I ponizej) Dwoma dlorimi nacisnac duzg komorp do momentu calkowite-go otwarcia rozrywalnego spa-wu pomipdzy dwoma komora-mi. (Patrz rysunek II ponizej) Caikowicie wymieszac roztwdr, wstrzasajac delikatnie worek. Roztwdr jest teraz gotowy do uzycia, a worek mczna zawie-sic na stojaku. (Patrz rysunek III ponizej)
Do kazdego z dwoch portdw dostepu mo2na podl^czyc linip dializy lub v/ymiany.
IV.a Jesli korzysta sie z dostppu typu Luer, usunaczatyczkp, odkrpcajacja i pociagajac, a nastppnie podtqczyc mpska koricowkp Luer Lock linii dializy lub wymiany do zeriskiej kori-cdwki na worku, wkrecajac jp i wciskaja.c. Upewnid sip, ze po-laczenie jest calkowite i szczel-ne. Teraz port jest otwarty. Sprawdzic, czy ptyn przeplywa swobodnie. (Patrz rysunek IV.a poniZej)
Gdy linie dializy lub wymiany bedp odtpczone od zlpcza typu Luer, polpczenie zostanie za-mknipte i przeptyw ptynu wstrzymany. Port typu Luer jest bezlglowy i ma mozliwodd pod-laczania dodatkowych przeply-wow w czasie dzialania,
IV.bW przypadku korzystania z portu do wstrzyknipd najpierw nalezy zdjac kapsel. Nastppnie wprowadzid grot przez gumowp przegrodp. Sprawdzic, czy plyn przeplywa swobodnie. (Patrz rysunek IV.b ponizej)
Nalezy uzywac wytpcznie z odpo-wiednimi urzadzeniami do wymiany pozanerkowej.
§RODKI OSTRO^NOSCI PRZY PRZECHOWYWANIU:
Wykazano chemicznp i fizycznp siabilnosc odtworzonego roztworu w cia.gu 24 godzin w temperaturze +22°C. Z chemlcznego punktu widzenia odtworzony roztwdr powinlen zostac niezwlocznie uzyty. Jesli nie zostanie zuzyty od razu. za czas i warunki przechowy-wania przed uzyciem odpowiada uiytkownik i normalnie czas ten nie powinien przekraczad 24 godzin lacznie z czasem zabiegu. Odtworzony roztwdr nie nadaje sip do powtornego uzycia. Usunac caly niezuzyty roztwdr bezposrednio po
THIS PAGE IS INTENTIONALLY LEFT BLANK
Prismasol and Gambro are trademarks belonging to the Gambro Group
c
C£
CO
<
JJ !*&
s»|
m

Prismasof U
UK, IE, MT Package leaflet: Information for the user.............................3
PL Ulotka dla pacjenta..............................................................5
X ................... ............X
UK, IE, MT The following information is intended
for healthcare professionals only.........................................7
PL Informacje przeznaczone wyt^cznie dla personelu
medycznego lub pracownikow stuzby zdrowia....................9
“gambro
with pin 2013-0
THIS PAGE IS INTENTIONALLY LEFT BLANK
PrismasoP 4- mmol/l Potassium Solution for haemodialysis/ haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrale/ glucose monohydrate/ lactic acid solution 90% wlw / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• if you have any further questions, ask your doctor, pharma-
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
WHAT IS IN THIS LEAFLET:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. WHAT PRISMASOL IS AND WHAT IT IS USED FOR
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate. glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltra-tion (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be
used in case of drug poisoning with dialysable or filterable substances. Prismasol 4 mmol/l Potassium is indicated particularly in patients who are norrnokalaemic (a normal concentration of potassium in the blood).
2. WHAT YOU NEED TO KNOW BEFORE YOU ARE GIVEN 6 7
OTHER MEDICINES AND PRISMASOL
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hyper-calcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
PREGNANCY AND BREASTFEEDING
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. As all medicines, your doctor will decide if you should Be given Prismasol if you are pregnant or breast-feeding.
DRIVING AND USING MACHINES
Prismasol is not known to affect your ability to drive or use ma-
3. HOW PRISMASOL IS USED
Always use this medicine exactly as your doctor or pharmacist has told you. Check wiih your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition
Calcium, Ca:" Magnesium. Mg7'
0.50
1.00
and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only".
IF YOU THINK YOU ARE GIVEN MORE PRISMASOL THAN YOU THINK YOU SHOULD BE Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure. Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately, if you have any further questions on the use of this medicine, please ask your doctor, pharmacist or
4. POSSIBLE SIDE EFFECTS Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hy-pophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration). Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. HOW TO STORE
PRISMASOL_
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
WHAT PRISMASOL CONTAINS The active substances are: Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
1000 ml of buffer solution (from the large compartment (B» contains Sodium chloride 6.450 g
Sodium hydrogen
After reconstitution:
The solutions In the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
Sodium, Na‘
Chloride,Cf 113.50 113.50
Hydrogen
Theoretical Osmolarity:
301 mOsm/l
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution:
7.0-8.5
WHAT PRISMASOL LOOKS LIKE AND CONTENTS OF THE PACK
Prismasol is presented in a two-compartment bag containing in the smaller compartment A. the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
MARKETING AUTHORISATION HOLDER
Gambro LundiaAB Magistratsvagen 16 SE-220 10 Lund SWEDEN MANUFACTURER Gambro Dasco S.p.A.
Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This laaflet was last revised in 07/2012
Prismasol3 4 mmol/l potasu Roztwor do hemodializy/ hemofiltracji
Wapnia chlorek dwuwodny/ magne-zu chlorek szesciowodny/ glukoza jednowodna/ kwasu rnlekowego roztwbr 90% w/w / sodu chlorek/ potasu chlorek/ sodu wodorowe-glan
NaleZy zapoznac sip z trescip ulotki przed przyjeciem leku, ponlewaz zawiera ona informacje wazne dla pacjenta.
• Nalezy zachowac te ulotkp, aby w razie potrzeby mic ja ponow-nie przeczytac,
• Nalezy zwrdcic sip do lekarza. farmaceuty lub pielegniarki w razie jakichkolwiek dalszych watpliwoSci.
• Jesli rasllj sie kiorykolwiek z objawow niepoZadanych lub wystppip jakiekolwiek objawy niepoZadane, w fym niewy-mienlone w ulotce, nalezy powiedziec o tym lekarzowi, farmaceucie lub pielegniarce.
SPIS TRE§CI ULOTKI:
1. Go to jest Prismasol 4 mmol/l potasu i w jakim celu sie go stosuje
2. Informacje wazne przed przyjpciem roztworu Prismasol 4 mmol/l potasu
3. Jak stosowac Prismasol 4 mmol/l potasu
4. Mozliwe dzialania niepozpdare
5. Jak przechowywac Prismasol 4 mmol/l potasu
6. Zawartosc opakowania i inne informacje
1. CO TO JEST
PRISMASOL 4 MMOL/L POTASU IW JAKIM CELU Sll= GO STOSUJE_
Prismasol zawiera substancje czynne: wapnia chlorek dwuwodny, magnezu chlorek szesciowod-ny, glukoza jednowodna, kwasu mlekowego roztwor 90% w/w, sodu chlorek. potasu chlorek, sodu wodorowpglan.
Prismasol 4 mmol/l potasu jest sto-sowany w leczeniu nlewydolnosci nerek Jako roztwor do hemofiltracji lub hemodiafiltracji (jako roztwor zastppczy przy utracie pfynow z krwi przeplywajpcej przez filtr) oraz cipglej hemodializy lub hemodiafiltracji (krew przeplywa po jednej stronie membrany dializacyjnej. podczas gdy roztwdr do hemodializy przeplywa po drugiej stronie membranyj.
Prismasol 4 mmol/l potasu mozna rowniez stosowac w przypadku zatrucia lekami zawierajacymi substancje ulegajace dializie lub filtracji.
Prismasol 4 mmol/l potasu jest wskazany u pacjentbw, majacych normalne stezenie potasu we krwi.
2. INFORMACJE WAZNE PRZED PRZYJECIEM ROZTWORU PRISMASOL 4 MMOL/L 8 9
rem lekarza wykwalifikowanego w leczeniu niewydolnodci nerek przez zastosowanie hemofiltracji, hemodiafiltracji i ciaglej hemodializy. Przed zastosovvaniem leczenia i podczas niego zostanie zbadana krew pacjenta, np, rownowaga kwasowo-zasadowa i stezenie elektrolitow (soli we krwi).
Nalezy doktadnie monitorowac stp-zenie glukozy we krwi, zwtaszcza gdy paejent choruje na cukrzyce. INNE LEKII
PRISMASOL 4 MMOL/L POTASU
NaleZy powiedziec lekarzowi lub farmaceucie o wszystkich lekach przyjmowanych obecnie lub ostat-nio a takze o lekach, ktore paejent planuje przyjmowac.
Stezenie we krwi innych przyjmowanych lekow moze zostac obnizone podczas leczenia. Lekarz zdecyduje, czy wymagana jest zmiana przyjmowanych Iskow.
W szczegblnosci nalezy powiedziec lekarzowi o przyjmowaniu nast^pu-jacych lekow:
• glikczydow (sfosowanych w leczeniu okreslonych chorob serca), gdyz podczas hipokalie-mii (obnizonego stgzenia potasu we krwi) zwiekszaja one ryzyko wystgpienia zaburzeh rytmu serca (nieregularnego lub przy-spieszonego bicia serca);
• witaminy D i produktbw lecz-niezyeh zawieraj^cych wapri, gdyz moga one zwi?kszac ryzyko wysta.pienia hiperkalce-mii (wysokiego stezenia wapnia we krwi);
• jakichkolwiek dodatkow wodo-roweglanu sodu obecnych w innych lekach, gdyz moze on zwiekszac ryzyko wyst^pienia zasadowicy metabolieznej (nadmiaru wodoroweglanow
CIAZAI KARMIENIE PIERSIA
W ci^zy i w okresie karmienia pier-siq lub gdy istnieje podejrzenie, ze kobieta jest w ciazy, lub gdy planuje oiqze, przed zasiosowaniem lego leku nalezy poradzid si§ lekarza lub farmaceuty.
Tak jak w przypadku wszystkich lekdw. lekarz zadecyduje o poda-waniu roztworu Prismasol kobietom w ci^Zy lub karmi^cym piersia.
1000 ml roztworu elektrolitowego (mala komora A) zawiera:
Wapnia chlorek dwuwodny 5.145 g
Teoretyczna osmolamosc: 301 mOsm/l
mmol/l mEq/l
........ 1,75 3,50
0,50 1,00
140,00 140.00 113,50 113,50
3.00 3,00 >3- 32.00 32,00
4.00 4.00 6,10
PROWADZENIE POJAZDOW I OBSLUGA MASZYN
Nie wiadomo, czy Prismasol wplywa na zdolnodd prowadzenia pojazddw i obstugi maszyn.
3. JAK STOSOWAd PRISMASOL 4 MMOUL
POTASU_
Ten lek nalezy zawsze stosowad zgodnie z zateceniami lekarza.
W razie w?tpliwosci nalezy zwrocic sip do lekarza lub farmaceuty. Obj?tosc uzywanego roztworu Prismasol 4 mmol/l potasu zalezv od stanu klinicznego pacjenta i docelowego bllansu plyndw. Dlatego decyzj? o obj?tosci dawki podejmuje lekarz odpowiedzialny za leczenie.
Droga podawania: do podawania dozylnego i hemodializy.
Instrukcje uZycia znajduja. si? w cz?dci ..Informacje przeznaczone wyiqcznie dla fachowego personelu medycznego”.
GDY PACJENTOWI WYDAJE SIE. ZE PODANO MU WI^KSZA ILOSC ROZTWORU PRISMASOL NIZ ZALECANA
Nalezy doktadnie monitorowac bilans plynow, rdwnowag? elektrolitow? I kwasowo-zasadowa. Przedawkowanie moZe spowodo-wac przeciazenie plynaml u pacjen-tow z nievvydolnoscia. nerek. Przedawkowanie moZe prowadzic do powaznych nast?pstw, takich jak zastoinowa niewydolnosc serca, zaburzenia rownowagi elektrolito-wej czy kwasowo-zasadowej.
Cl?gle stosowanie hemofiltracji usuwa nadmiar plynu i elektrolitow. W przypadku przewodnienia nalezy zwiekszyc ultrafiltracj? i zmniejszyc wielkosc przeplywu roztworu do hemofiltracji. W przypadku cipz-klego odwodnienia konieczne jest przerwanie ultrafiltracji I odpowied-nle zwipkszenie naplywu roztworu do hemofiltracji.
W razie jakichkolwiek dalszych watpliwosci zwigzanych ze stoso-waniem tego leku nalezy zwrocic si? do lekarza, farmaceuty lub pielpgniarki.
4. MOZLIWE DZIALANIA
NIEPOZADANE_
Jak kaZdy lek, lek ten moze powo-dowac dzialania niepozpdane, cho-ciaz nie u kaZdego one wystgpia.
Mozliwe s? nastepujace dzialania niepozadane zwiazane z uzywa-niem roztworu: przewodnienie lub odwodnienie (nieprawidlowo wysoka lub niska obj?tosc wody w organizmie), zaburzenia elektro-litowe (sole mineralne we krwi), hipofosfatemia (nieprawidlowo niskie st?zenie fosforanow we krwi). hiperglikemia (nieprawidlowo wysokie stezenie glukozy we krwi) i alkaloza metaboliczna (proces, ktdry powoduje gldwnie zwi?ksze-nie st?Zenia wodoroweglanu w osoczu).
Moga wystapid pewne reakcje niepozadane zwiazane z dializa, np. nudnosci, wymioty, skurcze mi?sni oraz nisdocisnienie (niskie cisnienie krwi).
Jesli wystapia jakiekoiwiek objawy niepozadane, w tym wszelkie moZliwe objawy niepozadane niewymienione w ulotoe, naleZy zwrocid si? do lekarza, farmaceuty lub piel?gniarki.
5. JAK PRZECHOWYWAC PRISMASOL 4 MMOUL POTASU_
Lek naleZy przechowywad w miej-scu niewidocznym i niedost?pnym dla dzieci.
Nie przechowywad w temperaturze poniZej +4°C.
Nie stosowad tego leku po uplywie terminu waZnosci zamieszczonego na etykiecie i opakowaniu. Termin waZnosci oznacza ostatni dziert danego mlesiaca.
6. ZAWARTOSd OPAKOWANIA I INNE INFORMACJE _
CO ZAWIERA
PRISMASOL 4 MMOL/L POTASU Substancje czynne:
Pned odtworzeniem/zmiesza-
Magnezu chlorek
Glukoza bezwodna 22,000 g
1000 ml roztworu buforowego (duZa komora B) zawiera:
Sodu wodorow?glan 3,090 g
Po odtworzeniu/zmieszaniu: Roztwory w komorach A (250 ml) i B (4750 ml) sa mieszane w celu otrzymania jednego odtworzonego/ zmieszanego roztworu (5000 ml) skladajacego si? z:
Waph, Ca-' Magnez, Mg:-Sod, Na-Chlorki, Cl Mteczan Wodoroweglan, Potas, K‘ Glukoza
Inne skiadnlki leku to: dwutlenek wegla, woda do wstrzykiwah. pH odtworzonego/zmieszanego roztworu wynosi: 7,0 do 8,5
JAK WYGLADA
PRISMASOL 4 MMOUL POTASU I CO ZAWIERA OPAKOWANIE
Prismasol 4 mmol/l potasu jest pakowany w dwukomorowe work! zawierajace w mniejszej komorze A roztwor elektrolitow, a w wi?kszej komorze B - roztwor buforowy. Ostateczny odtworzony/zmieszany roztwor uzyskuje si? po rozerwa-niu spawu i wymieszania obu roztworow. Odtworzony/zmieszany roztwor jest przezroczysty i lekko zotty. Kazdy worek (A+B) zawiera 5000 ml roztworu do hemofiltracji i hemodializy, Kazdy worek umieszczony jest w przezroczystym opakowaniu zewnetrznym.
W kaZdym opakowaniu znajduj? si?
dwa worki i ulotka informacyjna.
PODMIOT ODPOWIEDZIALNY I
WYTWORCA
Gambro Lundia AB
Magistratsvagen 16
SE-220 10 Lund
SZWECJA
PRODUCENT
Gambro Dasco S.p.A.
Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
WtOCHY
Data ostatniej aktualizacji ulotki: 07/2012
The following information is intended for healthcare professionals only
PrismasoP 4 mmol/l Potassium Solution for haemodialysis/ haemofiltration PRECAUTIONS
Carefully follow the instructions for use/handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37'C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must
of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and Haemodialysis solution may cause sepsis and shock. METHOD OF ADMINISTRATION
Intravenous use and for haemodi-
substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (postdilution).
POSOLOGY
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance, The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and
adolescents: 500 - 3000 ml/hour Children: 15-35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and
adolescents: 500 - 2500 ml/hour Children: 15-30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 551. INSTRUCTIONS FOR HANDLING The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged.
All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/ or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5). Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
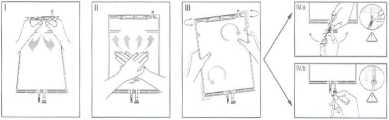
I Immediately Before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the Small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use. and can be hung on the equipment. (See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports,
IV.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag; tighten. Using thumb and fingers, break the blue frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure IV.a below) IV.blf the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-renal replacement equipment.
STORAGE PRECAUTIONS
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately, if not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
n-PVC UK IE MT PL I
Y
Informacje przeznaczone wyt^cznie dla personelu medycznego lub pracownikow stuzby zdrowia
Prismasol® 4 mmol/l potasu Roztwbr do hemodializy/ hemofiltracji 6RODKI OSTROZNOSCI
Dokladnie przestrzegab instrukojl uzycia/post^powania.
Roztwor elektrolitow musi zostac zmieszany z roztworem bufo-rowym przed uzyciem w celu uzyskania koiicowego roztworu do hemofiltracji/hemodiafiltracji lub ciqglej hemodializy.
Nalezy starannie kontrolowac ogrzewanie roztworu do temperatu-ry ciala (+37“C), sprawdzajgc, czy roztwbr jest przezroczysty I wolny od czastek statych.
Nalezy dokladnie monitorowac kaliemie w celu umozliwienia prawidlowego wyboru najodpowied-niejszego stezenia potasu.
Nalezy regularnie mierzyc stcZenie nleorganicznych fosforanbw. W przypadku hipofosfatemil (niskie-go st$zenia fosforanbw we krwi), nieorganiczne fosforany nalezy uzupelniac
W przypadku braku rownowagi ply-now nalezy starannie monitorowac stan kliniczny pacjenta i przywracac normalny biians ptynow.
Uzycie zanieczyszczonych roztwo-rbw do hemofiltracji i hemodializy moze spowodowab posocznice i wstrz^s.
SPOSOB PODAWANIA
Do podawania dozylnego i hemodializy. Prismasol 4 mmol/l potasu uzywany jako roztwor zamienny jest podavvany do obwodu przed (predylucja) lub za hemofiltrem (postdylucja).
DAWKOWANIE
Objetosc uzywanego roztworu Prismasol 4 mmol/l potasu zalezy od stanu klinicznego pacjenta i docelowego bilansu ptynow. Dlatego decyzje o objotosci davvki podejmuje lekarz odpowiedzialny za leczenie.
Szybkosci przeplywu dla roztworu zamiennego w hemofiltracji i hemo-diafiltracji wynosza:
Dorosli i
mlodzieZ: 500 do 3000 ml/godz,
Dzieci: 15 do 35 ml/kg mc./godz. Szybkobci przeplywu dla roztworu do dializy (dializat) w ci^gtej he-modializle I ci^glej hemodiafiltracji wynoszg:
Dorosli I
mtodziez: 500 do 2500 ml/godz.
Dzieci: 15 do 30 ml/kg mc./godz. Najcz?sciej stosowane szybkosci przeplywu w przypadku doroslych wynoszq okolo 2000 ml/godz., co odpowiada dziennej obj^tosci wynosz^cej 55 I.
INSTRUKCJA POSTEjPOWANIA
Roztwor jest pakowany w dwuko-morowe worki.
Podczas podawania leku pacjen-towi nalezy stosowactechnik? aseptyczna..
Uzywac wylsicznie wtedy, gdy roztwor jest przezroczysty. a
kodzone. Wszystkie spawy muszq bye nienaruszone, W przypadku zauwazenia przecieku roztwor nalezy niezwlocznie wyrzucic. po-niewaz nie mozna zagwarantowac jalowosci.
Duza komora B wyposazona jest w port do wstrzykivvan. umoZliwiaj^cy dodanie po odfworzeniu/zmiesza-nlu do roztworu innych koniecznych lekbw. Lekarz pozostaje odpowie-dzlalny za okreslenie niezgodnosoi lekbw dodatkowych z roztworem Prismasol za pomoca sprawdzania zmiany barwy i (lub) wytwarza-nia osadu, nierozpuszczalnych kompleksbw lub krysztalow. Nalezy zapoznac si? z instrukcjg uzyeia dodawansgo leku.
Przed dodaniem leku nalezy sprawdzic. czy jest rozpuszczalny i stabilny w wodzie o pH takim jak roztwbr Prismasol 4 mmol/l potasu (pH odtworzonego/zmieszanego roztworu wynosi od 7,0 do 8.5).
Leki powinny bye dodawane do roztworu pod kontrolg lekarza w nastepujacy sposbb: Usungc plyn z portu do wstrzykniec, przytrzymac worek do gory nogaml, wstrzyknac lek przez port i starannie wymie-szab. Roztwbr nalezy podac niezwlocznie.
ipin20l3.03.iJ
Bezposrednio przez uzyciem zdjac zewnetrzne opakowanie z worka i wymieszac roztwory z dwoch rbZnych komdr. Uchwy-cic mat? komor? dtonrni i sci-skajac do momentu powslania otworu w rozrywalnym spawie oddzielajacym obydwie komo-ry. (Patrz rysunek I ponizej) Obiema dlonmi nacisn?d duza. komor? do momentu calkowite-go otwarcia rozrywalnego spa-wu pomi?dzy dwoma komora-ml. (Patrz rysunek II ponIZej) Calkowicie wymieszad roztwdr, wstrzasajac dellkalnle worek. Roztvvor jest teraz gotowy do uzycia, a worek moZna zawie-sic na stojaku. (Patrz rysunek III ponIZej)
Do kaZdego z dwoch portbw dost?pu moZna podl?czyc linie dializy lub wymiany.
IV.a W przypadku korzystanla ze zlacza typu Luer nalezy zdjac ostonke i podlaczyc meska koncowk? typu Luer linii dializy lub wymiany do Zehskiego lacznika Luer w worku, dokr?-cid. Chwytajac kciukiem i pal-cami odtamac niebieska lamli-wa zatyczke u podstawy I poru-szac nia tarn i z powrotem. Nie naleZy uzywac narzedzia. Nalezy upewnid si?, Ze zatyczka oddzielila si? calkowicie i Ze plyn moZe przeplywad swobod-nie. Zatyczka podczas terapli pozostaje w porcie Luer. (Patrz rysunek IV.a poniZej)
IV.bW przypadku korzystania z portu do wstrzykni?c najpierw nalezy zdjac kapsel. Nasi?pnie wprowadzic grot przez gumow? przegrod?. Sprawdzifi, czy plyn przeptywa sv/obodnie, (Patrz rysunek IV.b poniZej)
Nalezy uzywac wylqcznie z odpo-wiednlmi urzadzeniami do wymiany pozanerkowej.
$RODKIOSTROiNOSCI PRZY PRZECHOWYWANIU:
Wykazano chemiczna i fizyczna stabilnosc odtworzonego roztworu w ci?gu 24 godzin w temperaturze +22"C. Z chemicznego punktu widzenia odtworzony roztwdr powinien zostac niezwlocznie uZyty. Jesli nie zostanie zuZyty od razu. za czas i warunki przechowy-wania przed uzyciem odpowiada uZytkownik i normalnie czas ten nie powinien przekraczac 24 godziny Iqcznie z czasem zabiegu. Odtworzony roztwdr nie nadaje si? do powtornego uZycia. Usunac caly niezuZyty roztwor bezposrednio po
i-PVC UK It MT PI luer with pin 2013-03.indd
THIS PAGE IS INTENTIONALLY LEFT BLANK
Prismasol and Gambro are trademarks belonging to the Gambro Group

Package leaflet: information for the user
Prismasol 4 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How to use Prismasol
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 4 mmol/l Potassium is indicated particularly in patients who are normokalaemic (a normal concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 4 mmol/l Potassium in the following cases:
• Hyperkalaemia (a high concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure
treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine,can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium hydrogen carbonate 3.090 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
113.50 |
113.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
hco3- |
32.00 |
32.00 |
|
Potassium |
K+ |
4.00 |
4.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
301 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the frangible pin and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only:
Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock.
Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
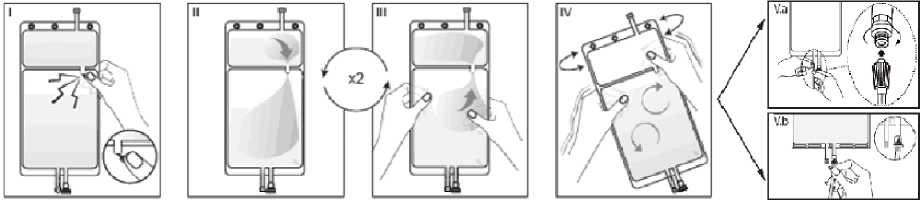
I Remove the overwrap from the bag immediately before use and discard any other packaging materials. Open the seal by breaking the frangible pin between the two compartments of the bag. The frangible pin will remain in the bag. (See figure I below)
II Make sure all the fluid from the small compartment A is transferred into the large compartment B. (See figure II below)
III Rinse the small compartment A twice by pressing the mixed solution back into the small compartment A and then back into the large compartment B. (See figure III below)
IV When the small compartment A is empty: shake the large compartment B so that the contents mix completely. The solution is now ready for use and the bag can be hung on the equipment. (See figure IV below)
V The dialysis or replacement line may be connected to either of the two access ports.
V.a If the luer access is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure V.a below)
When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
V.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure V.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22° C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
Package leaflet: information for the user
Prismasol 4 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 4 mmol/l Potassium is indicated particularly in patients who are normokalaemic (a normal concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 4 mmol/l Potassium in the following cases:
• Hyperkalaemia (a high concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure
treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.314 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
113.50 |
113.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
hco3- |
32.00 |
32.00 |
|
Potassium |
K+ |
4.00 |
4.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
301 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the frangible pin and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only:
Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Remove the overwrap from the bag immediately before use and discard any other packaging materials. Open the seal by breaking the frangible pin between the two compartments of the bag. The frangible pin will remain in the bag. (See figure I below)
II Make sure all the fluid from the small compartment A is transferred into the large compartment B. (See figure II below)
III Rinse the small compartment A twice by pressing the mixed solution back into the small compartment A and then back into the large compartment B. (See figure III below)
IV When the small compartment A is empty: shake the large compartment B so that the contents mix completely. The solution is now ready for use and the bag can be hung on the equipment. (See figure IV below)
V The dialysis or replacement line may be connected to either of the two access ports.
V.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or
replacement line to the female luer receptor on the bag: tighten. Using thumb and fingers, break the blue frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure V.a below)
V.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure V.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22° C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
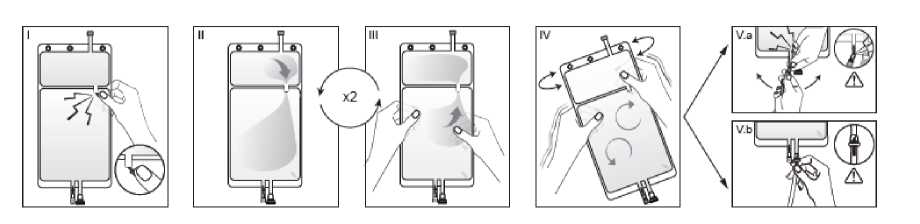
Package leaflet: information for the user
Prismasol 4 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 4 mmol/l Potassium is indicated particularly in patients who are normokalaemic (a normal concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 4 mmol/l Potassium in the following cases:
• Hyperkalaemia (a high concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
other . Your
Tell your doctor or pharmacist if you are given, have recently been given or might be given any medicines.
The blood concentration of some of your other medicines may be reduced during the treatment doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium hydrogen carbonate 3.090 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
113.50 |
113.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
hco3- |
32.00 |
32.00 |
|
Potassium |
K+ |
4.00 |
4.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
301 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only:
Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour 15 - 35 ml/kg/hour
Children:
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap with a twist and pull motion, and connect the male luer lock on the dialysis or replacement line to the female luer receptor on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely. (See figure IV.a below).
When the dialysis or replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needle-less and swabbable port.
IV.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
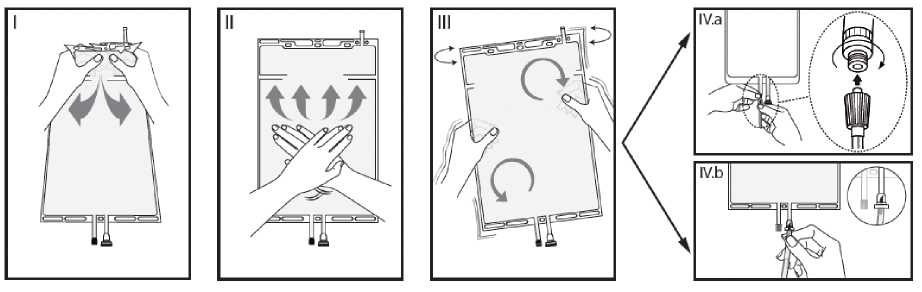
Package leaflet: information for the user
Prismasol 4 mmol/l Potassium Solution for haemodialysis/haemofiltration
Calcium chloride dihydrate/ magnesium chloride hexahydrate/ glucose monohydrate/ lactic acid solution 90% w/w / sodium chloride/ potassium chloride/ sodium hydrogen carbonate
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Prismasol is and what it is used for
2. What you need to know before you are given Prismasol
3. How Prismasol is used
4. Possible side effects
5. How to store Prismasol
6. Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the active substances calcium chloride dihydrate, magnesium chloride hexahydrate, glucose monohydrate, lactic acid solution 90% w/w, sodium chloride, potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for fluid lost from the blood passing through a filter) and continuous haemodialysis or haemodiafiltration (the blood flows on one side of a dialysis membrane while a haemodialysis solution flows on the other side of the membrane).
Prismasol solution may also be used in case of drug poisoning with dialysable or filterable substances.
Prismasol 4 mmol/l Potassium is indicated particularly in patients who are normokalaemic (a normal concentration of potassium in the blood).
2. What you need to know before you are given Prismasol
Do not use Prismasol 4 mmol/l Potassium in the following cases:
• Hyperkalaemia (a high concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
Do not use haemofiltration/ dialysis in the following cases:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
• Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
Tell your doctor or pharmacist if you are given, have recently been given or might be given any other medicines.
The blood concentration of some of your other medicines may be reduced during the treatment. Your doctor will decide if your medication should be changed.
In particular tell your doctor if you are using either of the following:
• Digitalis medicine (for treatment of certain heart conditions) as the risk of cardiac arrhythmia (irregular or rapid beating of the heart) caused by digitalis is increased during hypokalaemia (low concentration of potassium in your blood).
• Vitamin D and medicinal products containing calcium as they can increase the risk of hypercalcaemia (a high concentration of calcium in your blood).
• Any addition of sodium hydrogen carbonate found in other medicines, as it may increase the risk of metabolic alkalosis (excess of bicarbonate in your blood).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As all medicines, your doctor will decide if you should be given Prismasol if you are pregnant or breast-feeding.
Driving and using machines
Prismasol is not known to affect your ability to drive or use machines.
3. How Prismasol is used
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The volume of Prismasol used will depend on your clinical condition and the target fluid balance. The dose volume is therefore at the discretion of the responsible doctor.
Administration route: Intravenous use and for haemodialysis.
For instructions for use, please see section “The following information is intended for healthcare professionals only”.
If you think you are given more Prismasol than you think you should be
Your fluid balance, electrolyte and acid-base balance will be carefully monitored.
Overdose will result in fluid overload if you suffer from renal failure.
Overdose could lead to severe consequences, such as congestive heart failure, electrolyte or acid-base disturbances.
Continued application of haemofiltration will remove excess fluid and electrolytes. In case of hyperhydration, the ultrafiltration must be increased and the rate of administration of the solution for haemofiltration reduced. In the case of a severe dehydration it is necessary to cease ultrafiltration and to increase the inflow of solution for haemofiltration appropriately.
If you have any further questions on the use of this medicine, please ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hypophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration).
Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
5. How to store Prismasol
Keep this medicine out of the sight and reach of children Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
6. Contents of the pack and other information What Prismasol contains The active substances are:
Before reconstitution:
1000 ml of electrolyte solution (from the small compartment (A)) contains
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose anhydrous 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of buffer solution (from the large compartment (B)) contains
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.314 g
After reconstitution:
The solutions in the compartments A (250 ml) and B (4750 ml) are mixed to give one reconstituted solution (5000 ml) of which the composition is:
|
mmol/l |
mEq/l | ||
|
Calcium |
Ca2+ |
1.75 |
3.50 |
|
Magnesium |
Mg2+ |
0.50 |
1.00 |
|
Sodium |
Na+ |
140.00 |
140.00 |
|
Chloride |
Cl- |
113.50 |
113.50 |
|
Lactate |
3.00 |
3.00 | |
|
Hydrogen carbonate |
HCO3" |
32.00 |
32.00 |
|
Potassium |
K+ |
4.00 |
4.00 |
|
Glucose |
6.10 | ||
|
Theoretical Osmolarity: |
301 mOsm/l | ||
The other ingredients are: carbon dioxide, water for injections pH of the reconstituted solution: 7.0-8.5
What Prismasol looks like and contents of the pack
Prismasol is presented in a two-compartment bag containing in the smaller compartment A, the electrolyte solution, and in the larger compartment B, the buffer solution. The final reconstituted solution is obtained after breaking the peel seal and mixing both solutions. The reconstituted solution is clear and slightly yellow. Each bag (A+B) contains 5000 ml solution for haemofiltration and haemodialysis. The bag is overwrapped with a transparent film.
Each box contains two bags and a package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB Magistratsvagen 16 SE- 220 10 Lund SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last revised in 07/2012.
The following information is intended for healthcare professionals only:
Precautions:
Carefully follow the instructions for use / handling.
The electrolyte solution must be mixed with the buffer solution before use to obtain the reconstituted solution suitable for haemofiltration, haemodiafiltration or continuous haemodialysis.
Heating of the solution to body temperature (+37°C) must be carefully controlled verifying that the solution is clear and without particles.
Close monitoring of kalaemia must be carried out to enable the correct selection of the most appropriate potassium concentration.
The inorganic phosphate concentration should be measured regularly. Inorganic phosphate must be substituted in cases of low level of phosphate in the blood.
In case of fluid imbalance, the clinical situation must be carefully monitored and fluid balance must be restored.
The use of contaminated haemofiltration and haemodialysis solution may cause sepsis and shock. Method of administration:
Intravenous use and for haemodialysis. Prismasol, when used as a substitution solution is administered into the circuit before (pre-dilution) or after the haemofilter (post-dilution).
Posology:
The volume of Prismasol used will depend on the clinical condition of the patient and the target fluid balance. The dose volume is therefore at the discretion of the responsible physician.
Flow rates for the substitution solution in haemofiltration and haemodiafiltration are:
Adults and adolescents: 500 - 3000 ml/hour
Children: 15 - 35 ml/kg/hour
Flow rates for the dialysis solution (dialysate) in continuous haemodialysis and continuous haemodiafiltration are:
Adults and adolescents: 500 - 2500 ml/hour
Children: 15 - 30 ml/kg/hour
Commonly used flow rates in adults are about 2000 ml/h which correspond to a daily amount of 55 l. Instructions for handling:
The solution is packaged in a two-compartment bag.
Aseptic technique should be used throughout the administration to the patient.
Use only if the solution is clear and the overwrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
The large compartment B is fitted with an injection port for the possible addition of other necessary drugs after reconstitution of the solution. It is the responsibility of the physician to judge the compatibility of an additive medication with the Prismasol solution by checking for eventual colour change and/or eventual precipitation, insoluble complexes or crystals. The Instructions for Use of the medication to be added must be consulted.
Before adding a medication, verify it is soluble and stable in water at the pH of Prismasol (pH of reconstituted solutions is 7.0 to 8.5).
Medication should only be added to the solution under the responsibility of a physician in the following way: Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
I Immediately before use remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal between the two compartments. (See figure I below)
II Push with both hands on the large compartment until the peel seal between the two compartments is entirely open. (See figure II below)
III Ensure complete mixing of the solution by shaking the bag gently. The solution is now ready for use, and can be hung on the equipment. (See figure III below)
IV The dialysis or replacement line may be connected to either of the two access ports.
IV.a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or
replacement line to the female luer receptor on the bag; tighten. Using thumb and fingers, break the blue frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure IV.a below)
IV.b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure IV.b below)
Use only with appropriate extra-renal replacement equipment.
Storage precautions:
Chemical and physical in-use stability of the reconstituted solution has been demonstrated for 24 hours at +22°C. From a chemical point of view, the reconstituted solution shall be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user and shall not be longer than 24 hours including the duration of the treatment.
The reconstituted solution is for single use only. Discard any unused solution immediately after use.
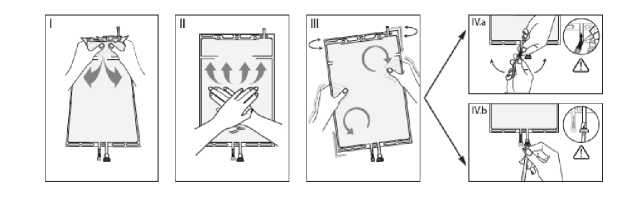
Prismasol 4 / UK / PL polyolefin luer connector with pin / 2012-07 6/6
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following undesirable effects related to the solution are conceivable: Hyper- or hypohydration (abnormally high or low volume of water in your body), electrolyte (salt in your blood) disturbances, hy-pophosphataemia (abnormally low concentration of phosphate in your blood), hyperglycaemia (abnormally high concentration of glucose in your blood) and metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration). Some undesirable effects related to the dialysis treatments can occur, such as nausea (feeling sick), vomiting (being sick), muscle cramps and hypotension (low blood pressure).
CO TO JEST
PRISMASOL 4 MMOL/L POTASU I W JAKIM CELU SIE GO STOSUJE_
Prismasol zawiera substancje czynne: wapnia chlorek dwuwodny, magnezu chlorek szedciowod-ny, glukoza jednowodna. kwasu mlekowego roztwor 90% w/w, sodu chlorek, potasu chlorek, sodu wodorow?glan.
Prismasol 4 mmol/l potasu jest sto-sowany w leczemu niewydolnosci nerek jako roztwor do hemofiltracji lub hemodiafiltracji (jako roztwor zastppczy przy utracie plynow z
POTASU_
NIE NALEZY
STOSOWAC ROZTWORU PRISMASOL 4 MMOUL POTASU, JESLI:
• u pacjenta wystgpuje hiperka-lemia (wysokie stQZenie potasu we krwi),
• u pacjenta wystgpuje alkaloza metaboliczna (proces. ktory po-woduje glownie wzrost stezenia wodoroweglanu w osoczu).
NIE STOSOWAC HEMOFILTRACJI/DIALIZY W NASTEPUJACYCH PRZYPADKACH:
• nlewydolnosc nerek z wyraZnym hiperkatabolizmem (niepra-widlowy wzrost katabolizmu), jedli objawdw uremii (objawy spowodowane wysokim stg-Zeniem mocznika we krwi) nie moZna ztagodzic za pomoca hemofiltracji:
• niewystarczajace cisnienle t?tni-cze w dostepie naczyniowym;
antykoagulacja ogolnoustrojowa (zmniejszona krzepliwosc krwi) przy zagrozeniu krwotokiem (krwawieniem).
OSTRZEZENIA I SRODKI OSTROZNOSCI
Przed rozpoczeciem przyjmowania roztworu Prismasol naleZy zwrdcid si$ do lekarza, farmaceuty lub pielegniarki.
Roztwdr powinien bye uzywany wytaeznie przez lub pod nadzo-
4. M02LIWE DZIALANIA
niepozadane_
Jak kaZdy lek. lek ten moze powo-dowac dziatania niepozadane. cho-ciaz nie u kazdego one wystppip.
PRISMASOL_
DO NOT USE PRISMASOL 4 MMOL/L POTASSIUM IN THE FOLLOWING CASES:
• Hyperkalaemia (a high concentration of potassium in your blood)
• Metabolic alkalosis (a process that primarily raises the plasma bicarbonate concentration)
DO NOT USE
HAEMOFILTRATION/ DIALYSIS IN THE FOLLOWING CASES:
• Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by high concentration of urea in your blood) cannot be corrected with haemofiltration,
• Insufficient arterial pressure in the vascular access,
Systemic anticoagulation (reduced clotting of your blood), if there is a high risk of haemorrhage (bleeding).
WARNINGS AND PRECAUTIONS
Talk to your doctor, pharmacist or nurse before you are given Prismasol.
The solution should be used only by, or under the direction of a doctor competent in renal failure treatments using haemofiltration, haemodiafiltration and continuous haemodialysis.
Before and during treatment, your blood condition will be checked, e.g. your acid-base balance and concentrations of electrolytes (salts in the blood).
Your blood glucose concentration should be closely monitored, especially if you are diabetic.
POTASU_
NIE NALEZY
STOSOWAC ROZTWORU PRISMASOL 4 MMOL/L POTASU. JE&LI:
• u pacjenta wystgpuje hiperka-lemia (wysokie stezenie potasu
• u pacjenta wyst^puje alkaloza metaboliczna (proces, ktory po-woduje glownie wzrost st§zenia wodoroweglanu w osoczu).
NIE STOSOWAC HEMOFILTRACJI/DiALIZY W NASTEPUJACYCH PRZYPADKACH:
• nlewydolnosc nerek z wyraznym hiperkatabolizmem (niepra-widlowy wzrost katabolizmu), jesli objawow uremii (objawy spowodowane wysokim sle-zeniem mocznika we krwi) nie mozna ztagodzic za pomoc^ hemofiltracji:
nlewystarczajgce ciSnienie t^tni-eze w dostepie naczyniowym;
» antykoagulacja ogolnoustrojowa (zmniejszona krzepliwosc krwi) przy zagrozeniu krwotokiem (krv/awieniem).
OSTRZEZENIA I SRODKI ostroZnoSci
Przed rozpoczedem przyjmovvania roztworu Prismasol nalezy zwrdcid sie do lekarza, farmaceuty lub pielegniarki.
Roztwor powinien bye uzywany wylaeznie przez lub pod nadzo-