Prostap Sr Dcs Solvent For Prolonged-Release Suspension For Injection In Pre-Filled Syringe
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
PROSTAP® SR DCS 3.75 mg Powder and Solvent for Prolonged-release Suspension for Injection in Pre-filled Syringe
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
PROSTAP SR Powder: contains 3.75 mg leuprorelin acetate (equivalent to 3.57 mg base).
Sterile Solvent: Each ml contains carmellose sodium 5 mg, mannitol (E421) 50 mg, polysorbate 80 1 mg, acetic acid, glacial up to 0.05 mg in Water for Injections.
When reconstituted with Sterile Solvent, the suspension contains 3.75 mg/ml leuprorelin acetate.
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Powder and solvent for suspension for injection in pre-filled syringe
Powder: A sterile, lyophilised, white, odourless powder.
Solvent: A clear, odourless, slightly viscous, sterile solvent.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
(i) Metastatic prostate cancer.
(ii) Locally advanced prostate cancer, as an alternative to surgical castration.
(iii) As an adjuvant treatment to radiotherapy in patients with high-risk localised or locally advanced prostate cancer.
(iv) As an adjuvant treatment to radical prostatectomy in patients with locally advanced prostate cancer at high risk of disease progression.
(v) As neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced prostate cancer.
(vi) Management of endometriosis, including pain relief and reduction of endometriotic lesions.
(vii) Endometrial preparation prior to intrauterine surgical procedures including endometrial ablation or resection.
(viii) Preoperative management of uterine fibroids to reduce their size and associated bleeding.
In children:
Treatment of central precocious puberty (girls under 9 years of age, boys under 10 years of age).
(See Section 5.1)
4.2 Posology and method of administration
Posology
Prostate Cancer: The usual recommended dose is 3.75 mg presented as a one month depot injection and administered as a single subcutaneous or intramuscular injection every month. The majority of patients will respond to a 3.75 mg dose. PROSTAP SR therapy should not be discontinued when remission or improvement occurs. As with other drugs administered chronically by injection, the injection site should be varied periodically.
Response to PROSTAP SR therapy may be monitored by clinical parameters and by measuring serum levels of testosterone and acid phosphatase. Clinical studies with leuprorelin acetate have shown that testosterone levels increased during the first 4 days of treatment in the majority of non-orchidectomised patients. They then decreased and reached castrate levels by 2-4 weeks. Once attained, castrate levels were maintained as long as drug therapy continued. If a patient's response appears to be sub-optimal, then it would be advisable to confirm that serum testosterone levels have reached or are remaining at castrate levels. Transient increases in acid phosphatase levels sometimes occur early in the treatment period but usually return to normal or near normal values by the 4th week of treatment.
In patients treated with GnRH analogues for prostate cancer, treatment is usually continued upon development of castrate-resistant prostate cancer. Reference should be made to relevant guidelines.
Endometriosis: The recommended dose is 3.75 mg administered as a single subcutaneous or intramuscular injection every month for a period of 6 months only. Treatment should be initiated during the first 5 days of the menstrual cycle.
In women receiving GnRH analogues for the treatment of endometriosis, the addition of hormone replacement therapy (HRT - an estrogen and progestogen) has been shown to reduce bone mineral density loss and vasomotor symptoms. Therefore if appropriate, HRT should be co-administered with PROSTAP SR taking into account the risks and benefits of each treatment.
Endometrial preparation prior to intrauterine surgery: A single 3.75 mg subcutaneous or intramuscular injection 5-6 weeks prior to surgery. Therapy should be initiated during days 3 to 5 of the menstrual cycle.
Preoperative management of uterine fibroids: The recommended dose is 3.75 mg administered as a single subcutaneous or intramuscular injection every month, usually for 3-4 months but for a maximum of six months.
Elderly: As for adults.
Paediatric population:
The treatment of children with leuprorelin acetate should be under the overall supervision of the paediatric endocrinologist.
The dosing scheme needs to be adapted individually.
The recommended starting dose is dependent on the body weight.
Children with a body weight > 20 kg
1 ml (3.75 mg leuprorelin acetate) suspension of 44.1 mg sustained-release microcapsules in 1 ml vehicle solution are administered once a month as a single subcutaneous injection.
Children with a body weight <20 kg
In these rare cases the following dosage should be administered according to the clinical activity of the central precocious puberty:
0.5 ml (1.88 mg leuprorelin acetate) is administered once a month as a single subcutaneous injection.
The remainder of the suspension should be discarded. The child’s weight gain should be monitored.
Depending on the activity of the central precocious puberty, it may be necessary to increase the dosage in the presence of inadequate suppression (clinical evidence e.g.spotting or inadequate gonadotropin suppression in the LHRH test). The minimal effective monthly dose to be administered should then be determined by means of the LHRH test.
Sterile abscesses at the injection site often occurred when leuprorelin acetate was administered intramuscularly at higher than the recommended dosages. Therefore, in such cases, the medicinal product should be administered subcutaneously (see 4.4).
It is recommended to use the lowest volumes possible for injections in children in order to decrease the inconvenience which is associated with the intramuscular/subcutaneous injection.
The duration of treatment depends on the clinical parameters at the start of treatment or during the course of treatment (final height prognosis, growth velocity, bone age and/or bone age acceleration) and is decided by the treating paediatrician together with the legal guardian and, if appropriate, the treated child. The bone age should be monitored during treatment at 6-12 month intervals.
In girls with bone maturation of older than 12 years and boys with bone maturation of older than 13 years discontinuation of treatment should be considered taking into account the clinical parameters.
In girls, pregnancy should be excluded before the start of treatment. The occurrence of pregnancy during treatment cannot be generally excluded. In such cases, medical advice should be sought.
Note:
The administration interval should be 30 ± 2 days in order to prevent the recurrence of precocious puberty symptoms.
Method of administration
The pre-filled syringe of PROSTAP SR microsphere powder should be reconstituted immediately prior to administration by subcutaneous or intramuscular injection.
To prepare for injection, screw the plunger rod into the end stopper until the end stopper begins to turn.
Remember to check if the needle is tight by twisting the needle cap clockwise. Do not overtighten.
Holding the syringe upright, release the diluents by SLOWLY PUSHING the plunger until the middle stopper is at the blue line in the middle of the barrel.
NOTE: Pushing the plunger rod quickly or over the blue line will cause leakage of the suspension from the needle.
Gently tap the syringe on the palm keeping the syringe upright to thoroughly mix the particles to form a uniform suspension. The suspension will appear milky.
NOTE: Avoid hard tapping to prevent the generation of bubbles.
Remove the needle cap and advance the plunger to expel the air from the syringe.
At the time of injection, check the direction of the safety device (with round mark face up) and inject the entire contents of the syringe. Inject the entire contents of the syringe subcutaneously or intramuscularly as you would for a normal injection
Withdraw the needle from the patient. Immediately activate the safety device by pushing the arrow forward with the thumb or finger until the device is fully extended and a CLICK is heard or felt.
NOTE: The suspension settles out very quickly following reconstitution and therefore the product should be mixed and used immediately.
4.3 Contraindications
Hypersensitivity to the active substance, any of the excipients listed in section 6.1 or to synthetic gonadotrophin releasing homone (Gn-RH) or Gn-RH derivatives.
Women: PROSTAP SR is contra-indicated in women who are or may become pregnant while receiving the drug. PROSTAP SR should not be used in women who are breastfeeding or have undiagnosed abnormal vaginal bleeding.
Men: There are no known contra-indications to the use of PROSTAP SR in men.
In girls with central precocious puberty:
- Pregnancy and lactation
- Undiagnosed vaginal bleeding.
4.4 Special warnings and precautions for use
As would be expected with this class of drug, development or aggravation of diabetes may occur, therefore diabetic patients may require more frequent monitoring of blood glucose during treatment with PROSTAP SR.
Epidemiological data have shown that during androgen deprivation therapy changes in the metabolic condition (e.g. reduction in glucose tolerance or aggravation of preexisting diabetes) as well as an increased risk for cardiovascular diseases may occur. However, prospective data did not confirm the link between treatment with GnRH analogues and an increase in cardiovascular mortality. Patients at high risk for metabolic or cardiovascular diseases should be appropriately monitored.
Hepatic dysfunction and jaundice with elevated liver enzyme have been reported. Therefore, close observation should be made and appropriate measures taken if necessary.
Spinal fracture, paralysis and hypotension have been reported.
There is an increased risk of incident depression (which may be severe) in patients undergoing treatment with GnRH agonists, such as leuprorelin. Patients should be informed accordingly and treated as appropriate if symptoms occur.
Postmarketing reports of seizures have been observed in patients treated with leuprorelin acetate and these events have been reported in both children and adults, and in those with or without a history of epilepsy, seizure disorders or risk disorders for seizures.
Men: In the initial stages of therapy, a transient rise in levels of testosterone, dihydrotestosterone and acid phosphatase may occur. In some cases, this may be associated with a "flare" or exacerbation of the tumour growth resulting in temporary deterioration of the patient's condition. These symptoms usually subside on continuation of therapy. "Flare" may manifest itself as systemic or neurological symptoms in some cases.
In order to reduce the risk of “flare”, an anti-androgen may be administered beginning 3 days prior to leuprorelin acetate therapy and continuing for the first two to three weeks of treatment. This has been reported to prevent the sequelae of an initial rise in serum testosterone.
In the rare event of an abscess occurring at the injection site, testosterone level should be monitored as there may be inadequate absorption of leuprorelin from the depot formulation.
Patients at risk of ureteric obstruction or spinal cord compression should be considered carefully and closely supervised in the first few weeks of treatment. These patients should be considered for prophylactic treatment with anti-androgens. Should urological/neurological complications occur, these should be treated by appropriate specific measures.
Long-term androgen deprivation either by bilateral orchiectomy or administration of GnRH analogues is associated with increased risk of bone loss which, in patients with additional risk factors, may lead to osteoporosis and increased risk of bone fracture.
If an anti-androgen is used over a prolonged period, due attention should be paid to the contra-indications and precautions associated with its extended use.
Whilst the development of pituitary adenomas has been noted in chronic toxicity studies at high doses in some animal species, this has not been observed in long term clinical studies with leuprorelin acetate.
Androgen deprivation therapy may prolong the QT interval.
In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval (see section 4.5) physicians should assess the benefit risk ratio including the potential for Torsade de pointes prior to initiating PROSTAP SR.
Women: When considering the preoperative treatment of fibroids it is mandatory to confirm the diagnosis of fibroids and exclude an ovarian mass, either visually by laparoscopy or by ultrasonography or other investigative technique, as appropriate, before PROSTAP SR therapy is instituted.
During the early phase of therapy, sex steroids temporarily rise above baseline because of the physiological effect of the drug. Therefore, an increase in clinical signs and symptoms may be observed during the initial days of therapy, but these will dissipate with continued therapy.
The induced hypo-estrogenic state results in a small loss in bone density over the course of treatment, some of which may not be reversible. The extent of bone demineralisation due to hypo-estrogenaemia is proportional to time and, consequently, is the adverse event responsible for limiting the duration of therapy to 6 months. The generally accepted level of bone loss with LHRH analogues such as PROSTAP SR is 5%. In clinical studies with PROSTAP SR the levels varied between 2.3% and 15.7% depending on the method of measurement. During one six-month treatment period, this bone loss should not be important. In patients with major risk factors for decreased bone mineral content such as chronic alcohol and/or tobacco use, strong family history of osteoporosis, or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids, PROSTAP SR therapy may pose an additional risk. In these patients, the risks and benefits must be weighed carefully before therapy with PROSTAP SR is instituted. This is particularly important in women with uterine fibroids where age related bone loss may have already begun to occur.
Therefore, before using PROSTAP SR for the preoperative treatment of uterine fibroids, patients with major risk factors for decreased bone mineral content (see above) should have their bone density measured and where results are below the normal (5th percentile by DEXA scan) range, PROSTAP SR therapy should not be started.
In women with submucous fibroids there have been reports of severe bleeding following the administration of PROSTAP SR as a consequence of the acute degeneration of the fibroids. Patients should be warned of the possibility of abnormal bleeding or pain in case earlier surgical intervention is required.
PROSTAP SR may cause an increase in uterine cervical resistance, which may result in difficulty in dilating the cervix for intrauterine surgical procedures.
In women receiving GnRH analogues for the treatment of endometriosis, the addition of HRT (an estrogen and progestogen) has been shown to reduce bone mineral density loss and vasomotor symptoms.
Precautions
Men: Patients with urinary obstruction and patients with metastatic vertebral lesions should begin PROSTAP SR therapy under close supervision for the first few weeks of treatment.
Women: Since menstruation should stop with effective doses of PROSTAP SR, the patient should notify her physician if regular menstruation persists.
In girls with central precocious puberty: Before starting the therapy, a precise diagnosis of idiopathic and/or neurogenic central precocious puberty is necessary.
The therapy is a long-term treatment, adjusted individually. PROSTAP SR should be administered as precisely as possible in regular monthly periods. An exceptional delay of the injection date for a few days (30 ± 2 days) does not influence the results of the therapy.
In the event of a sterile abscess at the injection site (mostly reported after i.m. injection of higher than the recommended dosage) the absorption of leuprorelin acetate from the depot can be decreased. In this case the hormonal parameters (testosterone, oestradiol) should be monitored at 2-week intervals (see 4.2).
The treatment of children with progressive brain tumours should follow a careful individual appraisal of the risks and benefits.
The occurrence of vaginal bleeding, spotting and discharge after the first injection may occur as a sign of hormone withdrawal in girls. Vaginal bleeding beyond the first/second month of treatment needs to be investigated.
Bone mineral density (BMD) may decrease during GnRH therapy for central precocious puberty. However, after cessation of treatment subsequent bone mass accrual is preserved and peak bone mass in late adolescence does not seem to be affected by treatment.
Slipped femoral epiphysis can be seen after withdrawal of GnRH treatment. The suggested theory is that the low concentrations of estrogen during treatment with GnRH agonists weakens the epiphysial plate. The increase in growth velocity after stopping the treatment subsequently results in a reduction of the shearing force needed for displacement of the epiphysis.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
Since androgen deprivation treatment may prolong the QT interval, the concomitant use of PROSTAP SR with medicinal products known to prolong the QT interval or medicinal products able to induce Torsade de pointes such as class IA (e.g. quinidine, disopyramide) or class III (e.g. amiodarone, sotalol, dofetilide, ibutilide) antiarrhythmic medicinal products, methadone, moxifloxacin, antipsychotics, etc. should be carefully evaluated (see section 4.4).
4.6 Pregnancy and lactation
Safe use of leuprorelin acetate in pregnancy has not been established clinically.
Studies in animals have shown reproductive toxicity (see section 5.3). Before starting treatment with PROSTAP SR, pregnancy must be excluded. There have been reports of foetal malformation when PROSTAP SR has been given during pregnancy.
PROSTAP SR should not be used in women who are breastfeeding.
When used monthly at the recommended dose, PROSTAP SR usually inhibits ovulation and stops menstruation. Contraception is not ensured, however, by taking PROSTAP SR and therefore patients should use non-hormonal methods of contraception during treatment.
Patients should be advised that if they miss successive doses of PROSTAP SR, breakthrough bleeding or ovulation may occur with the potential for conception. Patients should be advised to see their physician if they believe they may be pregnant. If a patient becomes pregnant during treatment, the drug must be discontinued. The patient must be apprised of this evidence and the potential for an unknown risk to the foetus.
In girls with central precocious puberty: See section 4.3 Contraindications.
4.7 Effects on ability to drive and use machines
PROSTAP SR can influence the ability to drive and use machines due to visual disturbances and dizziness.
4.8 Undesirable effects
Adverse reactions seen with PROSTAP SR are due mainly to the specific pharmacological action, namely increases and decreases in certain hormone levels.
The following tables list adverse reactions with leuprorelin based on experience from clinical trials as well as from post-marketing experience. Adverse reactions are grouped by MedDRA System Organ Classes and frequency classification. Frequencies are defined as: very common (>1/10); common (>1/100 to <1/10); uncommon (>1/1,000 to <1/100); rare (>1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data).
Men: In cases where a "tumour flare" occurs after PROSTAP SR therapy, an exacerbation may occur in any symptoms or signs due to disease, for example, bone pain, urinary obstruction, weakness of the lower extremities and paraesthesia. These symptoms subside on continuation of therapy.
Tabulated list of adverse reactions
|
SOC |
Very common |
Common |
Uncommon |
Rare |
Very rare |
Not known |
|
Blood and lymphatic system disorders |
anaemia (reported in medicinal products of this class), thrombocytopaenia, leucopenia | |||||
|
Immune system disorders |
hypersensitivity reactions (including rash, pruritus, urticaria and rarely, wheezing or interstitial pneumonitis, anaphylactic reactions) | |||||
|
Metabolism and nutrition disorders |
weight fluctuation |
decreased appetite |
Lipids abnormal, glucose tolerance abnormal | |||
|
Psychiatric disorders |
insomnia, depression (see Section 4.4), mood changes (long-term use)** |
mood changes (short term use)** | ||||
|
Nervous system disorders |
headache (occasionaly severe) |
dizziness, parasthesiae |
pituitary apoplexy has been reported following initial |
paralysis (see Section 4.4), seizure |
|
administration in patients with pituitary adenoma | ||||||
|
Eye disorders |
visual impairment | |||||
|
Cardiac disorders |
palpitations, electrocardiogram QT prolonged (see Sections 4.4 and 4.5) | |||||
|
Vascular disorders |
hot flush |
pulmonary embolism, hypertension, hypotension (see Section 4.4) | ||||
|
Gastrointestinal disorders |
nausea |
diarrhoea, vomiting | ||||
|
Hepatobiliary disorders |
hepatic function abnormal, liver function test abnormal (usually transient) |
jaundice | ||||
|
Skin and subcutaneous tissue disorders |
hyperhydrosis | |||||
|
Musculoskeletal, connective tissue and bone disorders |
muscle weakness, bone pain |
arthralgia |
myalgia, weakness of lower extremities |
spinal fracture (see Section 4.4), reduction in bone mass which may occur with the use of GnRH agonists | ||
|
Renal and urinary disorders |
urinary tract obstruction | |||||
|
Reproductive system and breast disorders |
Libido decreased, erectile dysfunction, testicular atrophy |
gynaecomastia | ||||
|
General disorders and administration site conditions |
Fatigue, injection site reaction, e.g., induration, erythema, pain, abscesses, swelling, nodules, ulcers and necrosis |
oedema peripheral |
pyrexia |
** mood changes (long term use: frequency of ‘common’ and short term use: frequency of ‘uncommon’
Women: Those adverse events occurring most frequently with PROSTAP SR are associated with hypo-estrogenism; the most frequently reported are hot flushes, mood swings including depression (occasionally severe), and vaginal dryness. Estrogen levels return to normal after treatment is discontinued.
The induced hypo-estrogenic state results in a small loss in bone density over the course of treatment, some of which may not be reversible (see Section 4.4).
Vaginal haemorrhage may occur during therapy due to acute degeneration of submucous fibroids (see Section 4.4).
Tabulated list of adverse reactions
|
SOC |
Very common |
Common |
Uncommon |
Rare |
Very rare |
Not known |
|
Blood and lymphatic system disorders |
Anaemia (reported in medicinal products of this class), thrombocytopaenia, leucopenia | |||||
|
Immune system disorders |
hypersensitivity reactions (including rash, pruritus, urticaria and rarely, wheezing and interstitial pneumonitis, anaphylactic reactions) | |||||
|
Metabolism and nutrition disorders |
weight fluctuation |
decreased appetite, lipids abnormal |
glucose tolerance abnormal, which may affect diabetic control | |||
|
Psychiatric disorders |
insomnia |
mood altered depression (see Section 4.4) | ||||
|
Nervous system disorders |
headache (occasionally severe) |
parasthesiae, dizziness |
pituitary haemorrhage has been reported following initial administration in patients with pituitary adenoma |
paralysis (see Section 4.4), seizure | ||
|
Eye disorders |
visual impairment | |||||
|
Cardiac disorders |
palpitations | |||||
|
Vascular disorders |
hot flush |
pulmonary embolism, hypertension, hypotension (see Section 4.4) | ||||
|
Gastrointestinal disorders |
nausea |
diarrhoea, vomiting | ||||
|
Hepatobiliary disorders |
liver function test abnormal (usually |
hepatic function abnormal, jaundice |
|
transient) | ||||||
|
Skin and subcutaneous tissue disorders |
hair loss | |||||
|
Musculoskeletal, connective tissue and bone disorders |
arthralgia, muscle weakness |
myalgia |
spinal fracture (see section 4.4), reduction in bone mass which may occur with the use of GnRH agonists | |||
|
Reproductive system and breast disorders |
breast tenderness, breast atrophy, vulvovaginal dryness |
vaginal haemorrhage | ||||
|
General disorders and administration site conditions |
Oedema peripheral, injection site reaction e.g.injection site induration, erythema, pain, abscesses, swelling, nodules, ulcers and necrosis |
pyrexia, fatigue |
In Children: In the initial phase of therapy, a short-term increase as flare-up of the sex hormone level occurs, followed by a decrease to values within the pre-pubertal range. Due to this pharmacological effect, adverse events may occur particularly at the beginning of treatment.
Tabulated list of adverse reactions
|
SOC |
Very common |
Common |
Uncommon |
Rare |
Very rare |
Not known |
|
Immune system disorders |
Hypersensitivity (fever, rash, e.g. itching, anaphylactic reactions) | |||||
|
Psychiatric disorders |
emotional lability | |||||
|
Nervous system disorders |
headache |
pituitary haemorrhage following initial administration in patients with pituitary adenoma |
seizure | |||
|
Gastrointestinal disorders |
abdominal pain / abdominal cramps, nausea/vomiting |
|
Skin and subcutaneous tissue disorders |
acne | |||||
|
Reproductive system and breast disorders |
vaginal haemorrhage, spotting**, vaginal discharge | |||||
|
General disorders and administration site conditions |
injection site reactions |
** In general, the occurrence of vaginal spotting with continued treatment (subsequent to possible withdrawal bleeding in the first month of treatment) should be assessed as a sign of potential underdosage. The pituitary suppression should then be determined by an LHRH test
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No case of overdose has been reported.
In animal studies, doses of up to 500 times the recommended human dose resulted in dyspnoea, decreased activity and local irritation at the injection site. In cases of overdose, the patients should be monitored closely and management should be symptomatic and supportive.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Gonadotrophin Releasing Hormone Analogues
ATC code: L02AE 02
PROSTAP SR contains leuprorelin acetate, a synthetic nonapeptide analogue of naturally occurring GnRH which possesses greater potency than the natural hormone. Leuprorelin acetate is a peptide and therefore unrelated to the steroids. Chronic administration results in an inhibition of gonadotrophin production and subsequent suppression of ovarian and testicular steroid secretion. This effect is reversible on discontinuation of therapy.
Administration of leuprorelin acetate results in an initial increase in circulating levels of gonadotrophins which leads to a transient increase in gonadal steroid levels in both men and women. Continued administration of leuprorelin acetate results in a decrease of gonadotrophin and sex steroid levels. In men serum testosterone levels, initially raised in response to early luteinising hormone (LH) release, fall to castrate levels in about 2-4 weeks. Estradiol levels will decrease to postmenopausal levels in premenopausal women within one month of initiating treatment.
The drug is well absorbed from the subcutaneous or intramuscular route, binds to luteinising hormone releasing hormone (LHRH) receptors and is rapidly degraded. In this dose form, an initial high level of leuprorelin acetate in the plasma is achieved within 3 hours followed by a drop over 24-48 hours to maintenance levels of 0.3-0.8ng/ml and a slow decline thereafter. Effective levels persist for 30-40 days after a single dose.
Leuprorelin acetate is inactive when given orally.
A randomised, open-label, comparative multi-centre study was performed to compare the efficacy and safety of the 3.75 mg and 11.25 mg depots of leuprorelin acetate. 48% of patients included had locally advanced disease (T3N0M0), 52% of patients had metastatic disease. Mean serum testosterone level fell below the threshold for chemical castration (0.5 ng/ml) at one month of treatment, continuing to decrease thereafter and stabilising at a value below the castration threshold. The decline in serum PSA mirrored that of serum testosterone in both groups.
In an open, prospective clinical trial involving 205 patients receiving 3.75 mg leuprorelin acetate on a monthly basis as treatment for metastatic prostate cancer, the long-term efficacy and safety of leuprorelin acetate was assessed. Testosterone levels were maintained below the castrate threshold over the 63-month follow up period. Median survival time exceeded 42.5 months for those receiving monotherapy and 30.9 months for those receiving leuprorelin acetate in combination with anti-androgens (this difference relating to baseline differences between groups)
In a meta-analysis involving primarily patients with metastatic disease, no statistically significant difference in survival was found for patients treated with LHRH analogues compared with patients treated with orchidectomy.
In another randomised, open-label, multi-centre comparative trial, leuprorelin acetate in combination with flutamide has been shown to significantly improve disease-free survival and overall survival when used as an adjuvant therapy to radiotherapy in 88 patients with high-risk localised (T1-T2 and PSA of at least 10 ng/mL or a Gleason score of at least 7), or locally advanced (T3-T4) prostate cancer. The optimum duration of adjuvant therapy has not been established. This US study used a higher dose of leuprorelin acetate (7.5mg/month) which is therapeutically equivalent to the European licensed dose.
The use of a LHRH agonist may be considered after prostatectomy in selected patients considered at high risk of disease progression. There are no disease-free survival data or survival data with leuprorelin acetate in this setting.
Neoadjuvant leuprorelin acetate prior to radiotherapy has been shown to reduce prostate volume.
In children:
Reversible suppression of pituitary gonadotropin release occurs, with a subsequent decrease in oestradiol (E2) or testosterone levels to values in the pre-pubertal range.
Initial gonadal stimulation (flare-up) may cause vaginal bleeding in girls who are already post-menarchal at start of treatment. Withdrawal bleeding may occur at the start of treatment. The bleeding normally stops as treatment continues.
The following therapeutic effects can be demonstrated:
- Suppression of basal and stimulated gonadotropin levels to pre-pubertal levels.
- Suppression of prematurely increased sexual hormone levels to pre-pubertal levels and arrest of premature menstruation;
- Arrest/involution of somatic pubertal development (Tanner stages);
- Improvement/normalisation of the ratio of chronological age to bone age;
- Prevention of progressive bone age acceleration;
- Decrease of growth velocity and its normalization;
- Increase in final height.
Treatment result is the suppression of the pathologically, prematurely activated hypothalamic-pituitary-gonadal axis according to pre-pubertal age.
In a long-term clinical trial in children treated with leuprorelin at doses up to 15mg monthly for > 4 years resumption of pubertal progression were observed after cessation of treatment. Follow up of 20 female subjects to adulthood showed normal menstrual cycles in 80% and 12 pregnancies in 7 of the 20 subjects including multiple pregnancies for 4 subjects.
5.2 Pharmacokinetic properties
Studies submitted show that single intramuscular or subcutaneous doses of leuprorelin acetate over the dose range 3.75 to 15 mg results in detectable levels of leuprorelin acetate for more than 28 days, good bioavailability, a consistent and predictable pharmacokinetic profile, and biological efficacy at plasma levels of less than 0.5 ng/ml. The pharmacokinetic profile is similar to that seen in animal studies using the compound, with an initial high level of drug released from the microcapsules during reconstitution and injection followed by a plateau over a 2-3 week period before levels gradually become undetectable. There appears to be no significant difference between the routes of administration (im vs sc) in biological effectiveness or pharmacokinetics.
The metabolism, distribution and excretion of leuprorelin acetate in humans have not been fully determined.
In children:
Figure 1 presents leuprorelin serum levels after a single s.c. administration of leuprorelin acetate depot at a dosage of 30 pg/kg body weight. Peak serum levels are reached sixty minutes after administration (7.81 ± 3.59 ng/m)l. The AUC0.672 is 105.78 ± 52.40 ng x hr/ml.
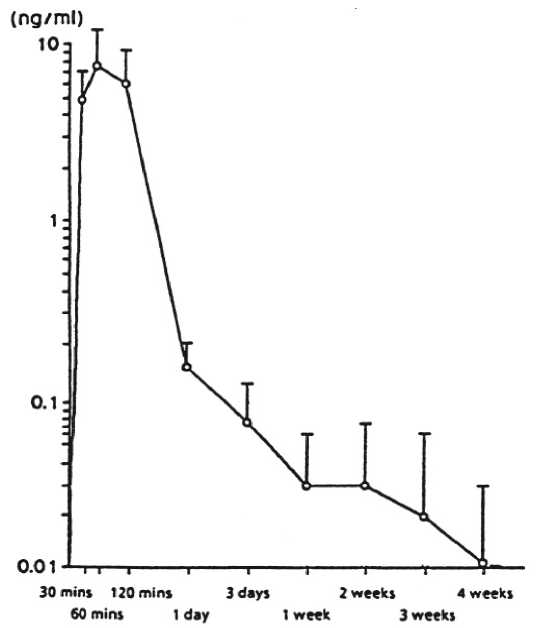
Figure 1: Leuprorelin serum levels after single s.c. administration of 30 pg/kg body weight of leuprorelin acetate as depot formulation (n=6) (Mean ± SD)
Preclinical safety data
5.3
A teratogenic effect has been observed in rabbits but not in rats.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
PROSTAP SR Powder Gelatin
Copoly (DL lactic acid/glycolic acid) 72:25 mol%
Mannitol (E421)
Sterile Solvent Carmellose sodium Mannitol (E421)
Polysorbate 80 Acetic acid, glacial Water for Injections
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years unopened.
Once reconstituted with sterile solvent, the suspension should be administered immediately.
6.4 Special precautions for storage
Do not store above 25 oC.
Do not refrigerate or freeze.
Store in the original container in order to protect from light.
6.5 Nature and contents of container
One dual chamber pre-filled syringe containing 3.75 mg leuprorelin acetate powder in the front chamber and 1 ml of Sterile Solvent in the rear chamber.
1 x 23 gauge syringe needle fitted with safety device.
1 x syringe plunger
6.6 Special precautions for disposal and other handling
Always ensure the safety device to prevent needle-stick injury is deployed after injection. Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Takeda UK Limited Building 3,
Glory Park,
Glory Park Avenue,
Wooburn Green,
BUCKS,
HP10 0DF
8 MARKETING AUTHORISATION NUMBER(S)
PL 16189/0012
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
28/04/2011
10 DATE OF REVISION OF THE TEXT
16/03/2016