Prostin E2 Alcoholic Solution 1Mg/Ml
date:25-OCT-07 13:32:58
o
I— O
cc
Pharmacia
Process Black P2385C eerste vol vc blokje: 20.5mm
5R9157
392
PHYSICIAN LEAFLET Presentation
Translucent, thixotropic gel containing 1 or 2 mg dinoprostone per 5 g (2.5 ml).
uses
Oxytocic. Prostin E2 Vaginal Cel is indicated for the induction of labour, when there are no fetal or maternal contra-indications.
Dosage and administration
In primigravida patients with unfavourable induction features (Bishop score of 4 or less), an initial dose of 2 mg should be administered vaginally. In other patients an initial dose of 1 mg should be administered vaginally.
In both groups of patients, a second dose of 1 mg or 2 mg may be administered after 6 hours as follows:
1 mg should be used where uterine activity is insufficient for satisfactory progress of labour.
2 mg may be used where response to the initial dose has been minimal.
Maximum dose 4 mg in unfavourable primigravida patients or 3 mg in other patients (see "Precautions").
The syringe should be assembled by following the sequence in the diagram.
The gel should be inserted high into the posterior fornix avoiding administration into the cervical canal. The patient should be instructed to remain recumbent for at least 30 minutes.
Contra-Indications, warnings, etc.
Contra-indications: Prostin E2 Vaginal Cel should not be used where the patient is sensitive to prostaglandins.
Prostin E2 Vaginal Cel is not recommended in the following circumstances:
1. For patients in whom oxytocic drugs are generally contra-indicated or where prolonged contractions of the uterus are considered inappropriate such as: Cases with a history of Caesarean section or major uterine surgery;
Cases where there is cephalopelvic disproportion;
Cases in which fetal malpresentation is present;
Cases in which there is clinical suspicion or definite evidence of pre-existing fetal distress;
Cases in which there is a history of difficult labour and/or traumatic delivery; Grand multiparae with six or more previous term pregnancies.
2. Patients with ruptured membranes.
3. In patients with a past history of, or existing, pelvic inflammatory disease, unless adequate prior treatment has been instituted.
4. In patients where there is clinical suspicion or definite evidence of placenta praevia or unexplained vaginal bleeding during this pregnancy.
5. Patients with active cardiac, pulmonary, renal or hepatic disease.
Interactions with other medicaments and other forms of interaction: Since it has been found that prostaglandins potentiate the effect of oxytocin, it is not recommended that these drugs are used together. If used in sequence, the patient's uterine activity should be carefully monitored.
Effects on ability to drive and to use machines: Not applicable.
Other undesirable effects: The most commonly reported events are vomiting, nausea and diarrhoea. Certain rare events that should be especially noted are: hypersensitivity to the drug; uterine rupture; and cardiac arrest. Other adverse effects, reported in decreasing order of severity are:
Pulmonary/amniotic fluid embolism;
Abruptio placenta;
Stillbirth, neonatal death;
Uterine hypercontractility or hypertonus;
Fetal distress;
Hypertension - systemic (maternal);
Bronchospasm/asthma;
Rapid cervical dilation;
Fever;
Backache;
Rash;
Vaginal symptoms - warmth, irritation, pain.
In addition, other adverse reactions that have been seen with the use of prostaglandin E2 for term labour induction have included: uterine hypercontractility with fetal bradycardia; uterine hypercontractility without fetal bradycardia; and low Apgar scores in the newborn.
PATIENT INFORMATION LEAFLET
Prostin® E2
Vaginal Cels 1 mg and 2 mg
(dinoprostone)
This leaflet gives you a summary of Information about your medicine. Please read it carefully before or immediately after you are given Prostin E2 vaginal Gel. The leaflet cannot tell you everything about your medicine, if you have any questions or are not sure about anything, ask your doctor or midwife. Keep this leaflet; you may want to read it again.
What Is In Prostin E2 Vaginal Gel?
Prostin E2 Vaginal Gel 1 mg contains 1 mg of the active ingredient, dinoprostone, in 3 grams of a thick, almost clear gel. Prostin E2 Vaginal Cel 2 mg contains 2 mg of the active ingredient, dinoprostone, in 3 grams of a thick, almost clear gel. The gel also contains the inactive ingredients triacetin and colloidal silicon dioxide (anhydrous), to which some patients may occasionally be allergic.
Prostin E2 Vaginal Gel is packed in syringes that contain either 1 mg or 2 mg of dinoprostone in 3 grams of gel. These syringes are packed singly and are for single use only.
Who makes Prostin E2 vaginal Gel?
Prostin E2 Vaginal Gel is made by Pfizer Manufacturing Belgium NV, Rijksweg 12, B-2870 Puurs, Belgium. The company authorised to sell this medicine in the UK is Pharmacia Limited, Ramsgate Road, Sandwich, Kent, CT13 9NJ, UK.
How does Prostin E2 vaginal Gel work?
Prostin E2 is a prostaglandin. Prostaglandins produced naturally in the body are very important in spontaneous labour. Prostin E2 Vaginal Gel is used to start off (induce) labour in normal pregnant women, particularly when the baby is healthy and ready to be born. This means that the medicine will help your uterus (womb) to start contracting and you will go into labour. Giving you the gel makes your uterus start to contract in exactly the same way as if you had gone into labour without any help.
Is Prostin E2 Vaginal Gel right for you?
Most women can be treated with Prostin E2 Vaginal Gel. Some women may need extra checks during treatment and for some women a different treatment may be better.
Your doctor or midwife will ask you questions before giving you Prostin E2 Vaginal Gel to make sure it is safe for you. If you do not understand any of the questions, ask your doctor or midwife to explain. You should not be given this medicine if you have had an allergic reaction, such as a rash, swelling, wheezing or breathing problems after being given dinoprostone, any other prostaglandin or any of the other ingredients in the gel, which are listed above.
Prostin E2 Vaginal Cel is not recommended if:
° Your doctor thinks that to start off or increase labour would be unsuitable for you, for example:
- if you have had a Caesarean section or any major surgery to your womb.
- if the size of your baby's head means there may be a problem in he or she being delivered.
- if there has been or there is suspected fetal distress (your baby is short of oxygen).
- if you had a difficult labour or traumatic delivery in a previous pregnancy.
- if you have already had six or more full-term pregnancies.
- the baby is not lying with his or her head down.
° Your waters have broken.
° You have an infection of your womb, ovaries or tubes (pelvic inflammatory disease) unless you are receiving treatment for this, or if you have ever had such an infection in the past.
° You have been told that you have, or might have, placenta praevia (where the placenta lies across the entrance to the womb, rather than being high up and out of the way during birth), which causes bleeding from the vagina during pregnancy and may require that your baby is delivered by Caesarean section.
° During your pregnancy you have had bleeding from the vagina and the cause of the bleeding is not known.
° if you have a current heart, lung, kidney or liver disease, if you answer yes to any of the following questions or you are not sure, tell your doctor Immediately. Your doctor may want to monitor you more closely or he or she may decide that another treatment would be better for you or your baby.
° Do you have glaucoma (raised pressure in the eye)?
° Do you have asthma or have you ever suffered from asthma?
0 Do you have epilepsy or have you ever had an epileptic fit?
° Have you had, or been told you had, abnormally strong contractions of your womb during a previous labour?
0 Do you have scarring of your womb from a previous operation?
° Have you had high blood pressure (hypertension) at any time, including during this or any previous pregnancy?
Are you taking any other medicines?
Prostin E2 Vaginal Gel may interact with some medicines and cause problems. Tell your doctor if you are taking any medicines before you are given Prostin E2 Vaginal Gel. This includes medicines that you buy yourself.
Your doctor may give you antibiotics before you start this prostaglandin treatment. Prostin E2 Vaginal Cel can make you more sensitive to oxytocin, another medicine that strengthens the contractions of the womb. If you need to be given this medicine, your doctor or midwife will ensure that it is not given at the same time as Prostin E2 Vaginal Gel, and he or she will monitor your contractions very carefully.
|
code |
guidelines |
dimensions |
date |
country |
|
5R9157 |
TSE-I002G |
105x580/35 |
19-JUN-07 EA |
UNITED |
Use in pregnancy and lactation: Prostin E2 Vaginal Cel is only used during pregnancy, to induce labour.
Prostaglandins are excreted in breast milk. This is not expected to be a hazard given the circumstances in which the product is used.
o
U1
tr
jj
>
Other special warnings and precautions: This product is available only to hospitals and clinics with specialised obstetric units and should only be used where 24-hour resident medical cover is provided.
Use the total contents of the syringe for one patient only. Discard after use. Use caution in handling this product to prevent contact with skin. Wash hands thoroughly with soap and water after administration.
Prostin E2 Vaginal Cel and Prostin E2 Vaginal Tablets are not bioequivalent.
Caution should be exercised in the administration of prostaglandin E2 in patients with:
(i) asthma or a history of asthma;
(ii) epilepsy or a history of epilepsy;
(iii) glaucoma or raised intra-ocular pressure;
(iv) compromised cardiovascular, hepatic, or renal function;
(v) hypertension.
As with any oxytocic agent, Prostin E2 Vaginal Cel should be used with caution in patients with compromised (scarred) uteri.
In labour induction, cephalopelvic relationships should be carefully evaluated before use of prostaglandin E2. During use, uterine activity, fetal status and the progression of cervical dilation should be carefully monitored to detect possible evidence of undesired responses, e.g. hypertonus, sustained uterine contractions, or fetal distress. In cases where there is a known history of hypertonic uterine contractility or tetanic uterine contractions, it is recommended that uterine activity and the state of the fetus should be continuously monitored throughout labour. The possibility of uterine rupture should be borne in mind where high-tone uterine contractions are sustained.
Animal studies lasting several weeks at high doses have shown that prostaglandins of the E and F series can induce proliferation of bone. Such effects have also been noted in newborn infants who have received prostaglandin E, during prolonged treatment. There is no evidence that short-term administration of prostaglandin Ez can cause similar bone effects.
Overdosage: Uterine hypertonus or unduly severe uterine contractions have rarely been encountered, but might be anticipated to result from overdosage. Where there is evidence of fetal distress or uterine hypertonus, then prompt delivery is indicated. Treatment of overdosage must be, at this time, symptomatic, since clinical studies with prostaglandin antagonists have not progressed to the point where recommendations may be made.
Pharmaceutical precautions
Store at 2-8°C. Keep out of reach and sight of children. The contents of one syringe to be used for one patient. Do not use after the expiry/use by date on the carton and syringe label. Discard after use.
Legal category
POM
Package quantities
Prostin E2 vaginal Cel is available in single packs of I mg or 2 mg.
Further Information
Unlike other oxytocics, prostaglandin E2 exhibits the capacity of the prostaglandins to influence uterine activity at any stage of gestation. Other Prostin E2 dosage forms are available for induction of labour (oral, vaginal and IV. routes), fetal death in utero (IV. route), therapeutic termination of pregnancy (IV. and extra-amniotic routes), missed abortion and hydatidiform mole (IV. route).
Marketing Authorisation numbers
PL 0032/0123 Prostin E2 Vaginal Cel 1 mg PL 0032/0124 Prostin E2 Vaginal Cel 2 mg
Holder of Marketing Authorisation
Pharmacia Limited, Ramsgate Road, Sandwich, Kent, CT13 9NJ, uk.
Date of preparation or last review
October 2007
® Prostin is a Registered Trademark:
METHOD OF ASSEMBLY OF THE SYRINGE
1. Remove from packaging.
2. Remove the syringe cap from the syringe.
^ t I
3. Insert syringe cap into barrel of syringe.
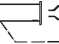

<
Being given Prostin E2 vaginal Gel
Prostin E2 Vaginal Cel can only be used in a hospital or a clinic with a specialist obstetric unit. Before you are given this medicine, you will be examined by your doctor or midwife. They need to know the position of your baby's head and how dilated (wide) your cervix (neck of the womb) is.
You will be given a numbered score after you have been examined. This is known as the Bishop score. The lower your Bishop score, the less ready you are to go into labour without any help. In this case, a higher dose of Prostin E2 Vaginal Cel is given.
Prostin E2 Vaginal Cel will be inserted into the posterior fornix (an area high up in your vagina) while you are lying down. You will then be asked to stay lying down for at least 30 minutes.
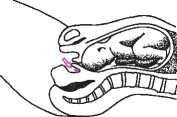
The usual dose is 1 mg. If you are primigravida (ie this is your first pregnancy) and you have a low Bishop score, you will be given 2 mg.
Your doctor or midwife may decide to give you a second dose of gel if you do not start having contractions or if you are only having weak contractions. Because this treatment can take a long time to have an effect in some women, your doctor or midwife will not give you a second dose until they are sure that this is needed. You should not have a second dose for at least six hours and many doctors and midwives will wait much longer than this. This means that you could even have your second dose the following day. You should not be given more than 4 mg.
Special points
Both you and your baby will be monitored when Prostin E2 Vaginal Cel has been given. The doctor will be checking your contractions, the well-being of your baby and the dilation of your cervix. If you are given the drug oxytocin before or after or at the same time as Prostin E2 Vaginal Cel, the gel may increase the effect of oxytocin, so the doctor will check the activity of your womb particularly carefully.
What if you are given too much Prostin E2 Vaginal Gel?
Tell your doctor or midwife if you think you have been given too much of this medicine. Symptoms of this would be excessive contractions of your womb (very strong, frequent and painful contractions) or severe side-effects such as feeling and being sick. If you have been given too much, your doctor may give you treatment to help.
Does Prostin E2 vaginal Gel have side-effects?
Most women who are given this medicine find that it causes them no problems. Women sometimes suffer from sickness or diarrhoea during treatment. These have seldom been bad enough for the woman to stop the treatment, if you have asthma, Prostin E2 Vaginal Cel could cause you to have an asthmatic attack. You must tell your doctor or midwife If you suffer from asthma or If you have difficulty breathing.
Because Prostin E2 Vaginal Cel makes the body start into labour in the same way as it would naturally, anything that can happen in a natural labour can also happen if you are taking Prostin E2 Vaginal Cel. Talk to your midwife or doctor about this if you want to know more, as they will be able to give you the information that you need.
Women having prostaglandin E2 vaginally, have reported the following:
- heart attack
- sudden blocking of a blood vessel by either amniotic fluid or blood dots;
- detached placenta
- stillbirth or neonatal death
- abnormally strong, frequent or long contractions of the womb
- itching and rash of the vaginal area
- slowing or quickening of the baby's heart rate and distress in the baby
- high blood pressure in the mother
- breathlessness
- feeling or being sick
- diarrhoea
- very quick opening of the cervix
- running a temperature
- backache
- rash
- baby born with an Apgar score lower than seven. (The Apgar score, which is measured on a scale of one to ten, is used to describe the baby's condition at birth. A low Apgar Score means that the baby's heart or lungs are not working properly.)
These have also been reported by women who have not taken prostaglandins.
If you have very long and strong contractions, the wall of the uterus could tear. (This can happen in natural labour too.) This is one reason for close monitoring during treatment.
Studies have shown proliferation (thickening) of bone in new-born infants who have been treated with prostaglandins for a long time. There is no evidence that this occurs following short-term treatment with Prostin E2 Vaginal Cel.
Tell your doctor or midwife if you have any problems with Prostin E2 Vaginal Cel, even if they are not mentioned here.
how to store Prostin E2 vaginal Gel
Process Black P2385C
The hospital pharmacy will store this medicine safely, at a temperature of 2-8°C. Keep out of reach and sight of children. Do not use after expiry/use by date on the carton and syringe label. Any out-of-date medicine will be disposed of safely.
Each syringe of Prostin E2 Vaginal Cel is for single use only and will be disposed of safely after use.
Remember: your medicine is for you. Never give it to others. It may harm them or their baby.
Date leaflet last revised: October 2007
6 Pharmacia Limited • Prostin is a Registered Trademark
Company Ref: PR 1_0
5R9157
|
code |
guidelines |
dimensions |
date |
country |
|
5R9157 |
TSE-I002G |
105x580/35 |
19-JUN-07 EA |
UNITED |