Prostin E2 Vaginal Gel 1Mg
|
Prostin® |
E2 Vaginal Gel 1 mg/2 mg |
|
Dinoprostone | |
|
iniiin | |
|
8R3729 69 |
PHYSICIAN LEAFLET
Prostin® E2 Vaginal Gel 1 mg/ 2 mg
Dinoprostone
Presentation
Translucent, thixotropic gel containing 1 or 2 mg dinoprostone per 3 g (2.5 ml).
Uses
Oxytocic. Prostin E2 Vaginal Gel is indicated for the induction of labour, when there are no fetal or maternal contra-indications.
Dosage and administration In primigravida patients with unfavourable induction features (Bishop score of 4 or less), an initial dose of 2 mg should be administered vaginally. In other patients an initial dose of 1 mg should be administered vaginally.
In both groups of patients, a second dose of 1 mg or 2 mg may be administered after 6 hours as follows:
1 mg should be used where uterine activity is insufficient for satisfactory progress of labour.
2 mg may be used where response to the initial dose has been minimal.
Maximum dose 4 mg in unfavourable primigravida patients or 3 mg in other patients (see "Precautions"). The syringe should be assembled by following the sequence in the diagram.
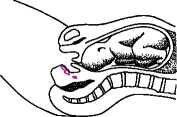
The gel should be inserted high into the posterior fornix avoiding administration into the cervical canal. The patient should be instructed to remain recumbent for at least 30 minutes.
Contra-indications, warnings, etc.
Contra-indications: Prostin E2 Vaginal Gel should not be used where the patient is sensitive to prostaglandins.
Prostin E2 Vaginal Gel is not recommended in the following circumstances:
1. For patients in whom oxytocic drugs are generally contra-indicated or where prolonged contractions of the uterus are considered inappropriate such as: Cases with a history of Caesarean section or major uterine surgery;
Cases where there is cephalopelvic disproportion; Cases in which fetal malpresentation is present; Cases in which there is clinical suspicion or definite evidence of pre-existing fetal distress;
Cases in which there is a history of difficult labour and/or traumatic delivery;
Grand multiparae with six or more previous term pregnancies.
2. Patients with ruptured membranes.
3. In patients with a past history of, or existing, pelvic inflammatory disease, unless adequate prior treatment has been instituted.
4. In patients where there is clinical suspicion or definite evidence of placenta praevia or unexplained vaginal discharge and/or abnormal bleeding during this pregnancy.
5. Patients with active cardiac, pulmonary, renal or hepatic disease.
The Clinician should be alert that the intracervical placement of dinoprostone gel may result in inadvertent disruption and subsequent embolization of antigenic tissue causing in rare circumstances the development of Anaphylactoid Syndrome of Pregnancy (Amniotic Fluid Embolism).
Interactions with other medicaments and other forms of interaction: Since it has been found that prostaglandins potentiate the effect of oxytocin, it is not recommended that these drugs are used together. If used in sequence, the patient's uterine activity should be carefully monitored.
Effects on ability to drive and to use machines:
Not applicable.
Other undesirable effects:
Cardiac disorders: Cardiac arrest
Vascular disorders: Hypertension
Gastrointestinal disorders:Diarrhoea, nausea, vomiting
General disorders and administration site conditions:
Fever
Immune system disorders: Hypersensitivity reactions such as anaphylactoid reactions and anaphylactic reactions including anaphylactic shock.
Musculoskeletal and connective tissue disorders: Back pain
Pregnancy, Puerperium and Perinatal conditions: Maternal-related conditions: uterine hypertonus, uterine rupture, abruptio placenta, pulmonary amniotic fluid embolism, rapid cervical dilatation Foetus-related conditions: uterine hypercontractility with/without fetal bradycardia fetal distress/altered fetal heart rate (FHR)
Neonatal conditions: neonatal distress, neonatal death, stillbirths, low Apgar score
Reproductive system and breast disorders: Warm feeling in vagina, irritation, pain Respiratory, thoracic and mediastinaldisorders: Asthma, bronchospasm
PATIENT INFORMATION LEAFLET
Prostin® E2 Vaginal Gel 1 mg/2 mg
Dinoprostone
Process Black P2385C
Read all of this leaflet carefully before you start taking this medicine.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, midwife or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, midwife or pharmacist.
In this leaflet:
1. What Prostin E2 Vaginal gel is and what it is used for
2. Before you are given Prostin E2 Vaginal gel
3. How Prostin E2 Vaginal gel is given to you
4. Possible side effects
5. How to store Prostin E2 Vaginal gel
6. Further information
1. What Prostin E2 Vaginal Gel is and what it is used for
Prostin E2 Vaginal Gel contain the prostaglandin dinoprostone and is used to "induce" labour. This means that the medicine will help your uterus (womb) to start contracting and you will go into labour. Dinoprostone is similar to the natural 'E2', type of prostaglandins which are made in your body when labour starts. Your doctor would have satisfied himself/herself that there are no conditions making the induction unsafe. It will only be given to you in a hospital or clinic which has an obstetric and maternity unit.
2. Before you are given Prostin E2 Vaginal Gel
Most women can be treated with Prostin E2 Vaginal Gel. Some women may need extra checks during treatment and for some women a different treatment may be better. Your doctor or midwife will ask you questions before giving you Prostin E2 Vaginal Gel to make sure it is safe for you. If you do not understand any of the questions, ask your doctor or midwife to explain.
Do not use Prostin E2 Vaginal gel:
• If you have had an allergic reaction (e.g.wheezing, breathlessness, swelling of the hands, face, itchy rash or redness of the skin) to dinoprostone or any other prostaglandin or any of the other ingredients in the gel, which are listed in Section 6 below.
Your doctor or midwife will not use Prostin E2 to start or strengthen your labour in certain circumstances if:
• you have heart, lung, kidney or liver disease
• you have had a Caesarean section or any major surgery to your womb.
• the size of your baby's head means there may be a problem with the delivery.
• there has been or there is suspected fetal distress (your baby is short of oxygen).
• you had a difficult labour or traumatic delivery in a previous pregnancy.
• you have already had six or more full-term pregnancies.
• your waters have broken.
• you have a past history or existing infection of your womb, ovaries or tubes (pelvic inflammatory disease) unless you are receiving treatment for these, or if you have ever had such an infection in the past.
• you have been told that you have or might have placenta praevia (where the placenta lies across the entrance to the womb, rather than being high up and out of the way during birth). This causes bleeding from the vagina during pregnancy and may require that your baby is delivered by Caesarean section.
• during your pregnancy you have had bleeding from
the vagina and the cause of the bleeding is not known.
• your baby is not lying with his or her head down.
Take special care with Prostin E2:
Tell your doctor or midwife if you have or have had in the past any of the following conditions as they may want to monitor you more closely.
• heart, lung, kidney or liver disease
• glaucoma (raised pressure in the eye)
• epilepsy
• suffered from asthma
• hypertension (high blood pressure) at any time, including during this or any previous pregnancy
• been told you had abnormally strong contractions of your womb during a previous labour
• scarring of your womb from a previous operation
• Are you 35 years or older?
• Is your pregnancy over 40 weeks?
• Do you have any complications related to this pregnancy?
Your doctor or midwife will ask you questions before giving you Prostin E2 Vaginal Gel to make sure they are safe for you.
If you do not understand any of the questions, ask your doctor or midwife to explain.
Taking other medicines:
Prostin E2 Vaginal Gel can make you more sensitive to another medicine called oxytocin which is used to strengthen contractions. Medical staff will normally try not to use this medicine at the same time as Prostin E2 Vaginal gel. If you need this medicine, your doctor or midwife will make sure they are not given to you close together and will watch over the contractions very carefully.
Please tell your doctor if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breastfeeding
Prostin E2 Vaginal gel will only be given to you in the late stages of pregnancy to induce labour.
Although prostaglandins are present in breast milk they will not harm your baby and you may breastfeed as normal after delivery.
Driving and using machinery
No effect on your ability to drive or use machinery is expected after being given Prostin E2 Vaginal gel
3. How Prostin E2 Vaginal gel is given to you
Prostin E2 Vaginal Gel can only be used in a hospital or a clinic with a specialist obstetric unit. Before you are given this medicine, you will be examined by your doctor or midwife. They need to know the position of your baby's head and how dilated (wide) your cervix (neck of the womb) is.
You will be given a numbered score after you have been examined. This is known as the Bishop score. The lower your Bishop score, the less ready you are to go into labour without any help. In this case, a higher dose of Prostin E2 Vaginal Gel is given.
Prostin E2 Vaginal Gel will be inserted into the posterior fornix (an area high up in your vagina) while you are lying down. You will then be asked to stay lying down for at least 30 minutes.
Skin and subcutaneous tissue disorders: Rash
Blood and lymphatic system disorders: An increased risk of post-partum disseminated intravascular coagulation has been described in patients whose labour was induced by pharmacological means, either with dinoprostone or oxytocin. The frequency of this adverse event, however, appears to be rare (<1 per 1,000 labours).
Post-marketing surveillance:
Use in pregnancy and lactation: Prostin E2 Vaginal Gel is only used during pregnancy, to induce labour
Prostaglandins are excreted in breast milk. This is not expected to be a hazard given the circumstances in which the product is used.
Other specialwarnings and precautions: This product is available only to hospitals and clinics with specialised obstetric units and should only be used where 24-hour resident medical cover is provided.
Use the total contents of the syringe for one patient only. Discard after use. Use caution in handling this product to prevent contact with skin. Wash hands thoroughly with soap and water after administration. Prostin E2 Vaginal Gel and Prostin E2 Vaginal Tablets are not bioequivalent.
Caution should be exercised in the administration of prostaglandin E2 in patients with:
i. asthma or a history of asthma;
ii. epilepsy or a history of epilepsy;
iii. glaucoma or raised intra-ocular pressure;
iv. compromised cardiovascular, hepatic, or renal function;
v. hypertension.
As with any oxytocic agent, Prostin E2 Vaginal Gel should be used with caution in patients with compromised (scarred) uteri.
In labour induction, cephalopelvic relationships should be carefully evaluated before use of prostaglandin E2. During use, uterine activity, fetal status and the progression of cervical dilation should be carefully monitored to detect possible evidence of undesired responses, e.g. hypertonus, sustained uterine contractions, or fetal distress.
In cases where there is a known history of hypertonic uterine contractility or tetanic uterine contractions, it is recommended that uterine activity and the state of the fetus should be continuously monitored throughout labour
The possibility of uterine rupture should be borne in mind where high-tone uterine contractions are sustained.
Animal studies lasting several weeks at high doses have shown that prostaglandins of the E and F series can induce proliferation of bone. Such effects have also been noted in newborn infants who have received prostaglandin E1 during prolonged treatment. There is no evidence that short-term administration of prostaglandin E2 can cause similar bone effects.
Women aged 35 years or older, those with complications during pregnancy and those with a gestational age over 40 weeks have been shown to have an increased risk of post-partum disseminated intravascular coagulation. In addition, these factors may further increase the risk associated with labour induction.
Therefore, in these women, use of dinoprostone should be undertaken with caution. Measures should be applied to detect as soon as possible an evolving fibrinolysis in the immediate post-partum phase.
Overdosage (symptoms,emergency procedures, antidotes): Overdosage may be expressed by uterine hypercontractility and uterine hypertonus. During use, uterine activity, fetal status and the progression of cervical dilation should be carefully monitored to detect possible evidence of undesired responses, e.g. hypertonus, sustained uterine contractions, or fetal distress. Because of the transient nature of PGE2-induced myometrial hyperstimulation, non-specific, conservative management was found to be effective in the vast majority of cases: i.e. maternal position change and administration of oxygen to the mother. If conservative management is not effective, B-adrenergic drugs may be used as a treatment of hyperstimulation following administration of PGE2 for cervical ripening, in appropriate patients.
Pharmaceutical precautions
Store at 2-8°C. Keep out of reach and sight of children. The contents of one syringe to be used for one patient. Do not use after the expiry/use by date on the carton and syringe label. Discard after use.
Legal category
POM
Package quantities
Prostin E2 Vaginal Gel is available in single packs of l mg or 2 mg.
Further information
Unlike other oxytocics, prostaglandin E2 exhibits the capacity of the prostaglandins to influence uterine activity at any stage of gestation. Other Prostin E2 dosage forms are available for induction of labour (oral, vaginal and IV. routes), fetal death in utero (IV. route), therapeutic termination of pregnancy (IV. and extra-amniotic routes), missed abortion and hydatidiform mole (IV. route).
Marketing Authorisation numbers
PL 00057/1029 PA 16/1/5 Prostin E2 Vaginal Gel 1 mg PL 00057/1025 PA 16/1/6 Prostin E2 Vaginal Gel 2 mg
Holder of Marketing Authorisation
Pfizer Limited, Ramsgate Road, Sandwich, Kent,
CT13 9NJ, UK.
Date of preparation or last review
02/2014
® Prostin is a Registered Trademark:
METHOD OF ASSEMBLY OF THE SYRINGE
1. Remove from packaging.
2. Remove the syringe cap from the syringe.
I I
3. Insert syringe cap into barrel of syringe.
|
-k- |
i | ||
|
\ ■ \ | |||
|
i |
- | ||
PR 4_0
Process Black P2385C
The usual dose is 1 mg. If this is your first pregnancy and you have a low Bishop score, you will be given 2 mg.
Your doctor or midwife may decide to give you a second dose of gel if you do not start having contractions or if you are only having weak contractions. Because this treatment can take a long time to have an effect in some women, your doctor or midwife will not give you a second dose until they are sure that this is needed. You should not have a second dose for at least six hours and many doctors and midwives will wait much longer than this. This means that you could even have your second dose the following day. You should not be given more than 4 mg.
Your doctor or midwife should be keeping a very close eye on you during your treatment. They should be able to act quickly if you have side-effects or if your womb reacts too strongly to the dose you are given.
Your doctor or midwife will do internal checks to make sure that your cervix is opening enough. They will also check your contractions (to make sure that they are not too strong) and your baby (to make sure he or she does not get distressed).
4. Possible side effects
Like all medicines Prostin E2 Vaginal gel can cause side effects, although not everybody gets them.
If you have asthma, Prostin E2 Vaginal gel could cause you to have an asthmatic attack. You must tell your doctor or midwife if you suffer from asthma or if you have difficulty breathing.
Rare side effects
Rare but serious side effects which can sometimes happen include the following:
• tearing or bursting of the wall of your womb (uterine rupture)
• heart attack
• allergic / anaphylactic reactions, including anaphylactic shock (serious allergic reactions which can include skin rash, itching, wheezing, shortness of breath, swollen face, lips, hands, fingers, neck and throat, sudden drop in blood pressure, abdominal pain and collapse ).
If you get any of these symptoms please tell your doctor or midwife straight away.
Common side effects
• vomiting (being sick)
• nausea (feeling sick)
• diarrhoea.
These have seldom been bad enough for the woman to stop the treatment.
Other side effects
As prostaglandins make the body go into labour in the same way as it would happen naturally, anything that can happen in a natural labour can also happen if you have been given Prostin E2 Gel. Talk to your midwife or doctor about this if you want to know more, as they will be able to give you the information that you need.
• sudden blockage of a blood vessel with amniotic fluid (the fluid which surrounds the baby) or by a blood clot in the lungs. This could cause chest pain and shortness of breath.
• placenta becoming detached
• stillbirth or death of the newborn baby
• abnormally strong, frequent or long contractions of the womb
• slowing or quickening of the baby’s heart rate and distress in the baby
• itching, soreness, rash or feeling of warmth of the vaginal area
• high blood pressure in the mother
• very quick opening of the cervix
• running a high temperature
• backache
• rash
• baby born with an Apgar score lower than seven.
(The Apgar score, which is measured on a scale of one to ten, is used to describe the baby’s condition at birth. A low Apgar Score means that the baby’s heart or lungs are not working properly.)
Studies have shown proliferation (thickening) of bone in new-born infants who have been treated with prostaglandins for a long time. There is no evidence that this occurs following short-term treatment with Prostin E2 Vaginal Gel.
A higher risk of a generalised bleeding disorder following delivery (post-partum disseminated intravascular coagulation-DIC) has been described in women who are aged 35 and above, whose pregnancies are more than 40 weeks and who have pregnancy-related complications.
If you think you may be having any of the above side effects, or you are worried about anything unusual happening during your labour, please tell your doctor or midwife.
5. How to store Prostin E2 Vaginal gel
The medicine will be kept out of the reach and sight of children.
Prostin E2 Vaginal gel will not be given to you after the expiry date. The expiry date refers to the last day of that month.
Your hospital pharmacist will store this medicine in a refrigerator at 2 to 8 °C before use.
6. Further information
What Prostin E2 Vaginal Gel contains:
The active substance is called dinoprostone.
The other ingredients are Triacetin and colloidal silicon dioxide.
What Prostin E2 Vaginal gel looks like and contents of the pack
Prostin E2 Vaginal gel is packed in syringes that contain 1 mg or 2 mg of dinoprostone in 3 grams of gel. Each pack contains one pre-filled syringe and the syringes are for single use only.
Marketing Authorisation Holder:
Pfizer Limited
Ramsgate Road
Sandwich
Kent
CT13 9NJ
UK
Manufacturer:
Pfizer Manufacturing Belgium NV Rijksweg 12 B-2870 Puurs Belgium
For further information on this medicine, please contact Pfizer Medical Information on: 01304 616161.
This leaflet was last revised in 02/2014
PR 4_0
8R3729