Pulmicort 100 Microgram Turbohaler
APPROVED
By Claudia Vincenzi at 3:47 pm, Sep 14, 2007
\
PULMICORT® 100 microgram TURBOHALER®
Powder for inhalation (budesonide) 200 metered actuations
Each actuation delivers 100 micrograms budesomde To be inhaled through your mouth into your lungs as directed by your doctor. Read enclosed leaflet before use
PL NO: 15814/0787 |-. Batch No: 01234567890
ECMA N0:027621010 Expiry: 1/1/2000
Do not store above 30°C. Replace the cover properly after use Keep out of the reach and sight of children.
It is important to use Pulmicort Turbohaler regularly Do not stop taking this medicine except on your doctor’s advice Pulmicort Turbohaler does not contains excipients
Pulmicort and Turbohaler are registered Trade Marks of AstraZeneca AB Sweden Manufactured by AstraZeneca AB Sodertalje. Sweden and is procured from within the EU and repackaged by the Product Licence Holder O P D Laboratories Ltd, Watford, Herts, WD2 4PR
PULMICORT®100 microgram TURBOHALER®
Powder for inhalation (budesonide) 200 metered actuations
Each ad nation delivers 100 n xerograms budesonide. To be inhaled through your mouth nto your lungs as dreded by your dodor Read enclosed leaflet before use II is important to use Pulmicort Turbohaler regularly Do not stop taking this medone except on your dodor's advice. Pulmicort Turbohaler does not contains excfxents
Do not store above 30°C Replace the cover properly after use i-1
PL NO: 15814/0787 Keep out of the reach and sight of children |POty
ECMA NO: 027621010 Batch No: 123456 Expiry: 1/1/2000
Pulmicort and Turbohaler are registered Trade Marks of AstraZeneca AB Sweden Manufactured by AstraZeneca AB Sodertal|e Sweden and is procured from within the EU and repackaged by the PL Holder OPD Laboratories Ltd Watford Herts WD2 4PR
|
tryjl C>f |
Oaf )r>oa label | ||
|
r |
> | ||
|
PULMICORT® | |||
|
100 microgram TURBOHALER® |
« o)^rv»nri | ||
|
(budesonide) | |||
|
<_ | |||
rv>r>0
Fud area To co'ie.r 0 f>\de^> of Irnpar teal Ccxr<har\
BUDESONIDE 100 microgram TURBOHALER
Powder for inhalation 200 metered actuations
Each actuation delivers 100 micrograms budesonide To be inhaled through your mouth into your lungs as directed by your doctor Read enclosed leaflet before use
PL NO: 15814/0787 |-. Batch No: 01234567890
ECMA N0:027621010 l^J Expiry: 1/1/2000
Do not store above 30°C Replace the cover properly after use Keep out of the reach and sight of children It is important to use Budesonide Turbohaler regularly Do not stop taking this medicine except on your doctor's advice Budesonide Turbohaler does not contains excipients Turbohaler is registered Trade Mark of AstraZeneca AB Sweden Manufactured by AstraZeneca AB, Sodertalje Sweden and is procured from within the EU and repackaged by the Product Licence Holder O P D Laboratories Ltd, Watford, Herts WD2 4PR
'rviro
rwv'v
BUDESONIDE 100 microgram TURBOHALER®
Powder for inhalation 200 metered actuations
Each actuation detvers 100 micrograms budesonrie To be inhaled through your mouth into your lungs as directed by your doctor. Read enclosed leaflet before use.
It is impodant to use Budesonide Turbohaler regularly. Do not slop talang this mediane except on your doctor's advice. Budesonrie Turbohaler does not contains excipients. Do not store above 30 °C Replace the cover property after use. i^i
PL NO: 15814/0787 Keep out of the reach and sight o( children. -
ECMA NO: 027621010 Batch No: 123456 Expiry: 1/1/2000
Turbohaler is a registered Trade Mark of AstraZeneca AB Sweden Manufactured by AstraZeneca AB Sodertal|e, Sweden and is procured from within the EU and repackaged by the PL Holder 0 P D Laboratories Ltd, Watford. Herts, WD2 4PR
^r
t rdt of Oierlcxbal
BUDESONIDE 100 microgram TURBOHALER®
BRAILLE LABEL
The Braille label will not obscure statutory information when overlabelled.
The Braille label will be placed on the back panel of the carton.
The Braille overlabel material will be of a quality and dot size as previously approved and the text will appear exactly as indicated on the mock up
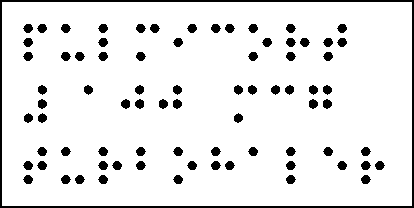
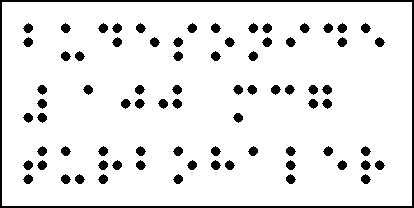
Keep out of the reach and sight of children.
200 METERED ACTUATIONS
' JSwder fcj jphalaticyp
Each actuation delivers * 100 miijqgrams budesonide
• •
To be inhaled through your mouth into your lungs as directed by your doctor. Read enclosed leaflet before use.
Do not store above 30°C. Replace the cover properly after use.
It is important to use Budesonide Turbohaler regularly.
Do not stop taking this medicine except on your doctor’s advice.
Budesonide Turbohaler does not contain excipients
• •
BN: XXXX <PIRY:dd/mm/yyyy LNo: 15814/0787 ECMA No: 027621010
BUDESONIDE I lOOmicrogram TURBOHALER®
Powder for inhalation
EAN Code 5 034188 xxxxxx
Place dispensing label here
Turbohaler is a registered Trade Mark of AstraZeneca AB, Sweden.
Manufactured by AstraZeneca AB, Sodertalje, Sweden and is procured from within the EU and repackaged by the Product Licence holder: O.P.D. Laboratories Ltd, Watford, Herts WD2 4PR
POM
Cxxx

• •
• • • • •
• • •
• 200 METERED
• • ACTUATIONS • • •
• ••
•PULMKX^RT® • 100microgram
•rJrbohaler®*.
• *(bude#oride) P«wder for inhalation • Eaate actuatior*delive*
• ••
|
100 microgfams |
• |
|
• • budesorwde |
• •• |
|
• • |
• |
|
• |
• • |
|
• |
• • |
|
• • • |
Keep out of the reach and sight of children.
To be inhaled through your mouth into your lungs as directed by your doctor. Read enclosed leaflet before use.
Do not store above 30°C. Replace the cover properly after use.
It is important to use Pulmicort Turbohaler regularly.
Do not stop taking this medicine except on your doctor’s advice.
POM
Pulmicort Turbohaler does not contain excipients
BN: XXXX <PIRY:dd/mm/yyyy LNo: 15814/0787 ECMA No: 027621010
PULMICORT® 100microgram TURBOHALER <
(budesonide) Powder for inhalation
EAN Code 5 034188 xxxxxx
Place dispensing label here
Pulmicort and Turbohaler are registered Trade Marks of AstraZeneca AB, Sweden.
Manufactured by AstraZeneca AB, Sodertalje, Sweden and is procured from within the EU and repackaged by the Product Licence holder: O.P.D. Laboratories Ltd, Watford, Herts WD2 4PR
|
POM | ||
200 METERED ACTUATIONS

Cxxx
(budesonide)