Qvar 100 Aerosol
<0
What is in this leaflet
1. What Qvar Aerosol is and what it is used for
2. What you need to know before you use Qvar Aerosol
DO NOT use Qvar Aerosol if you:
Warnings and precautions
Other medicines and Qvar Aerosol
Pregnancy and breast-feeding
D riving and using machines
3. How to use Qvar Aerosol
Qvar 50 Aerosol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is Qvar® 100 Aerosol but it will be referred as Qvar Aerosol throughout the leaflet. Please note that the leaflet also contains information about other strength, Qvar® 50micrograms Aerosol.
1. What Qvar Aerosol is and what it is used for
2. What you need to know before you use Qvar Aerosol
3. How to use Qvar Aerosol
4. Possible side effects
5. How to store Qvar Aerosol
6. Contents of pack and other information
Qvar Aerosol contains beclometasone dipropionate, which is one of a group of medicines known as corticosteroids.
Qvar Aerosol is used to prevent the symptoms of mild, moderate, or severe asthma, in patients who require regular treatment.
How your medicine works
• Qvar Aerosol works deep in your lungs to make breathing easier by reducing the inflammation, swelling and irritation in the airways. This type of medicine is known as a ‘preventer'. It needs to be taken regularly every day, even if you have no symptoms.
• This inhaler will not give immediate relief of wheezing or breathlessness during a sudden asthma attack. You will need to use a ‘reliever' inhaler, which contains a different medicine. You should still continue to use this inhaler.
• are allergic to beclometasone dipropionate or any of the other ingredients of this medicine (listed in section 6)
• are allergic to other similar inhalers
• are suffering from a sudden attack of breathlessness. It will not help. Use a quick-acting ‘reliever' inhaler for this purpose and carry it with you at all times.
Talk to your doctor before you start to take this
medicine if:
• you are suffering from tuberculosis (TB) now or have you suffered from it in the past.
• you must avoid alcohol for any reason.
• your asthma seems to be getting worse. Perhaps you are more wheezy and short of breath than usual, your ‘reliever' inhaler seems to be less effective, you require more puffs from your ‘reliever' inhaler than usual, or you do not seem to be getting better. Your doctor may need to increase the dose of your steroid inhaler or give you a course of steroid tablets, or change your treatment altogether. If you have an infection in your chest your doctor may prescribe a course of antibiotics.
• when transferring from steroid tablets to an inhaler you find that, even if your chest is getting better, you feel generally unwell, you develop a rash, eczema or a runny nose and sneezing (rhinitis). Do not stop treatment with your inhaler unless your doctor tells you to.
Important points to remember while you are using
this medicine:
• Your doctor may prescribe this inhaler to replace steroid tablets, which may mean for a short time you have to take both medicines. It is important to follow your doctor's advice. Whilst you are reducing the number of steroid tablets that you take you may feel generally unwell even though you can breathe as well as normal or better. If you have other allergies you may find that stopping your steroid tablets makes them worse. If this happens keep using your inhaler and tell your doctor.
• If you have been treated for a long time with high doses of inhaled steroid, you may require a course of steroid tablets or possibly a steroid injection in times of stress. For example, during admission to hospital after a serious accident, before an operation, during an acute attack of asthma or if you have a chest infection or other serious illness. Your doctor will decide if you need any extra steroid treatment and will also advise you as to how long you need to take the course of steroid tablets and how you should reduce these as you get better.
• There may be times when you need to take steroid tablets as well as using your inhaler, for example if you have worsening asthma attacks, you get a chest infection or you need an operation. Your doctor may give you a small supply of steroid tablets to be taken in these situations; if he does you will be given full instructions on how and when to take them. Contact your doctor immediately if you think that you need to take steroid tablets, even if you have your own supply.
• You should have been given a steroid card with this inhaler, if you have not, please ask your pharmacist for one. Make sure you carry your steroid card with you at all times until your doctor decides that it is no longer necessary.
• Visit your doctor regularly for a review of your condition.
• If you have to go into hospital, remember to take all your inhalers and other medicines with you.
• Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including any medicines bought without a prescription.
In particular tell your doctor if you are taking:
• Disulfiram.
• Metronidazole.
• If you are pregnant, planning to become pregnant or are breast-feeding, ask your doctor for advice before taking this medicine.
• Qvar Aerosol is not known to affect your ability to drive or operate machinery.
Important information about some ingredients of Qvar:
• Qvar contains a small amount of alcohol.
There are two strengths of Qvar aerosol available and your doctor will have chosen the strength which best suits your condition.
Remember that it is important to use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
This inhaler has a softer feel and a different taste than other inhalers you may have used before. This inhaler is an extra fine aerosol which results in more of each dose being delivered to your lungs. Your doctor may, therefore, prescribe a lower dose of this inhaler than your previously used inhaler.
Even when your doctor has prescribed a lower dose you may find that the number of puffs you are asked to take from this inhaler is the same as from your previous inhaler; this is because this inhaler may be a lower strength product (which means that each puff of this inhaler contains less beclometasone dipropionate than your old inhaler).
DO NOT take more puffs than your doctor has told
you to. In some circumstances, your doctor may prescribe more than the usual number of puffs. The usual number of puffs to take is:
Adults (including the elderly) and children over 12 years
• Mild Asthma
The starting dose is one puff twice a day. This may be increased up to two puffs twice a day.
• Moderate Asthma
The starting dose is two puffs twice a day. This may be increased up to four puffs twice a day.
• Severe Asthma
The starting dose is four puffs twice a day. This may be increased up to eight puffs twice a day.
The maximum dose is a total of sixteen puffs a day.
Qvar 100 Aerosol
Adults (including the elderly) and children over 12
years
• Mild to Moderate Asthma
The starting dose is one puff twice a day. This may be increased to two puffs twice a day.
• Severe Asthma
The starting dose is two puffs twice a day. This may be increased up to four puffs twice a day.
The maximum dose is a total of eight puffs a day.
Children under 12 years
Qvar Aerosol is not recommended for use in children under 12 years.
(-\
What to do if you think your treatment is not working
_V
If you think your usual treatment is not working, for example your symptoms are not getting better, or are getting worse, or you need to use more puffs from your reliever inhaler, or if your reliever inhaler does not seem to be working as well as usual, or your peak flow falls, please tell your doctor. Your asthma may be getting worse.
If you use more Qvar Aerosol than you should
If you forget to use Qvar Aerosol
If you stop using Qvar Aerosol
Using your Qvar Aerosol inhaler
Before use
How to use your inhaler
How to tell when your Qvar Aerosol device is empty
Cleaning instructions
4. Possible side effects
5. How to store Qvar Aerosol
It is important that you take your dose as stated on the pharmacist's label, or as advised by your doctor.
You should not increase or decrease your dose without seeking medical advice.
If you accidentally take more puffs than recommended, please tell your doctor.
If you forget to use this inhaler at your usual time, take your recommended number of puffs as soon as you remember unless it is nearly time to use your inhaler again. DO NOT take a double dose to make up for a forgotten dose. Then continue to use your inhaler regularly at the correct time, as prescribed by your doctor.
This inhaler must be used regularly, even when you feel well. You must not stop using your inhaler unless your doctor tells you to.
Ask your doctor for a prescription for a replacement inhaler before this one is empty.
If your doctor decides to stop treatment, return the inhaler to your doctor or pharmacist for safe disposal.
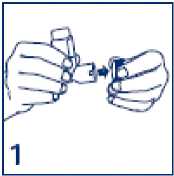
• If this is a new inhaler or if you have not used your inhaler for two weeks or more, it must be tested before use by removing the mouthpiece cover and pressing down on the canister inside the inhaler. Release 2 puffs into the air, away from you.
1. Take the cover off the mouthpiece.
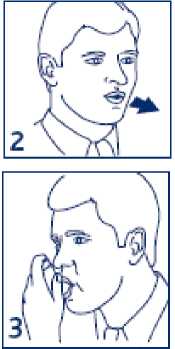
2. Breathe out as far as is comfortable and then immediately place the mouthpiece in your mouth and close your lips around it.
3. Start to breathe in slowly and deeply through your mouth and press down on the canister inside the inhaler as shown. This releases one puff of medicine. It is important that you carry on breathing in after the puff is released.
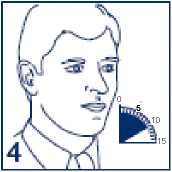
4. Hold your breath for 10 seconds, then breathe out slowly. If your doctor has prescribed more than one puff repeat steps 2 to 4 again. After use, replace the cover on the mouthpiece.
• Some people find it difficult to press their inhaler and breathe in at the same time.
A spacer device helps to overcome this problem. The spacer that fits Qvar Aerosol is called the AeroChamber Plus® spacer device. If you use the AeroChamber Plus® spacer device, please follow the instructions provided with it. Your doctor, nurse or pharmacist will be able to advise you about the AeroChamber Plus® device.
Alternatively your doctor may wish to prescribe the Qvar Autohaler® device which automatically releases a puff of medication as you breathe in.
When the canister is completely empty you will not feel or hear any propellant being discharged.
For normal hygiene, the mouthpiece of your inhaler should be cleaned weekly with a clean, dry tissue or cloth. You should also rinse your mouth with water after using your inhaler.
Do not wash or put any part of your inhaler in water
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If immediately after taking a puff of this inhaler, you feel wheezy or tight chested do not take any more puffs. Use your reliever inhaler to help your breathing and contact your doctor immediately.
Stop using your inhaler and tell your doctor immediately or go to the casualty department at your nearest hospital if the following happens:
• an allergic reaction (swelling of the lips, face or neck leading to severe difficulty in breathing; skin rash or hives).
This is a very serious but rare side effect. You may need urgent medical attention or hospitalisation.
Treatment with Qvar Aerosol may affect the normal production of corticosteroids in the body. Keep using your inhaler but see your doctor as soon as possible if you become unwell, particularly with any of the following:
• abdominal pain
• weakness
• vomiting
This is especially important if you have been exposed to other stress such as other illness, surgery, or infection.
The following side effects may also occur in patients taking beclometasone dipropionate. If you experience any of these effects, keep using your inhaler but see
your doctor if they last for a while or they are worrying
you:
• hoarseness
• a sore mouth or thrush (white spots in your mouth and throat). These are less likely if you rinse your mouth out with water after using your inhaler. If you get thrush your doctor may recommend a medicine to treat you
• feeling sick
• headache
• feeling dizzy or faint
• tremor
• change in taste
• increase in wheezing, shortness of breath and cough
• sleeping problems, depression or feeling worried, restless, nervous, over-excited or irritable. These effects are more likely to occur in children (Frequency not known).
At high doses, taken for prolonged periods the following side effects have been reported:
• bone thinning
• clouding of the lens of the eye (cataract) resulting in blurred vision
• loss of vision due to abnormally high pressure in the eye may occur.
Children or adolescents who are using the inhaler for a prolonged period may grow more slowly. Your doctor may therefore wish to monitor the height of a child receiving prolonged treatment with Qvar Aerosol.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at:www.mhra.gov.uk/yellowcard By reporting side effects, you can help provide more information on the safety of this medicine.
• Qvar Aerosol should not be stored above 25°C. Avoid storage in direct sunlight and heat. Protect from frost. You can use your inhaler at temperatures as low as -10°C
• Do not use Qvar Aerosol after the expiry date that is stated on the outer packaging. The expiry date refers to the last day of that month.
• The canister is pressurised and should not be punctured or burnt, even if it seems empty.
• If your doctor tells you to stop using the inhaler, please take it back to the pharmacist for safe disposal. Only keep the inhaler if your doctor tells you to.
• KEEP OUT OF SIGHT AND REACH OF CHILDREN.
• If your inhaler fails to work properly you should ask your doctor or pharmacist for advice.
• Medicines should not be disposed of via wastewater or household waste. If your doctor decides to stop treatment, return the inhaler to your doctor or pharmacist for safe disposal. These measures will help protect the environment.
/-\
6. Contents of pack and other information
Qvar Aerosol delivers your medicine as an aerosol spray for you to inhale directly into your lungs where it is needed.
What Qvar Aerosol contains
Qvar Aerosol contains 100 micrograms of the active ingredient beclometasone dipropionate in each actuation (puff).
Qvar Aerosol also contains the following:
Norflurane HFA-134a propellant and ethanol
What Qvar Aerosol looks like and contents of the pack:
Qvar Aerosol is a pressurised metered dose inhaler with a metal canister encased in an actuator (dark red plastic body and a white removable cap). The inhaler does not have a dose counter.
Canister contains 200 actuations.
Manufactured by: 3M Health Care Limited. 1 Morely Street, Loughborough, Leicestershire, UK.
OR
Laboratoires 3M Sante Avenue du 11 Novembre, pthiviers, France.
OR
Temmler Werke GmbH, Weihenstephanner Str. 28, 81673 Munchen, Germany.
Procured from within the EU and repackaged by the Product Licence holder: B&S Healthcare, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 0NU, UK. Qvar® 100 Aerosol
PL 18799/2623 [POM]
Leaflet date: 12.04.2016