Ranace 2.5Mg Capsules Hard
Package leaflet: Information for the user:
Ramipril 2.5 mg Capsules, hard Ramipril
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Ramipril Capsules are and what are they used for
2. What you need to know before you take Ramipril Capsules
3. How to take Ramipril Capsules
4. Possible side effects
5. How to store Ramipril Capsules
6. Contents of the pack and other information.
1. What Ramipril Capsules are and what are they used for
Ramipril belongs to the group of medicines called Angiotensin Converting Enzyme (ACE) inhibitors that act on the heart and blood vessels.
Ramipril works by:
• Decreasing your body’s production of substances that could raise your blood pressure
• Making your blood vessels relax and widen
• Making it easier for your heart to pump blood around your body.
Ramipril can be used:
• To treat high blood pressure (hypertension)
• To reduce the risk of you having a heart attack or stroke
• To reduce the risk or delay the worsening of kidney problems (whether or not you have diabetes)
• To treat your heart when it cannot pump enough blood to the rest of your body (heart failure)
• As treatment following heart attack (myocardial infarction) complicated with heart failure.
2. What you need to know before you take Ramipril Capsules
Do not take Ramipril Capsules:
• If you are allergic to ramipril, any other ACE inhibitor medicine or any of the ingredients of this medicine (listed in section 6). Signs of an allergic reaction may include rash, swallowing or breathing problems swelling of your face, lips, throat or tongue
• If you have ever had a serious allergic reaction called “angioedema”. The signs include itching, hives (urticaria), red marks on the hands, feet and throat, swelling of the throat and tongue, swelling around the eyes and lips, difficulty breathing and swallowing
• If you are having dialysis or any other type of blood filtration. Depending on the machine that is used ramipril may not be suitable for you.
• If you have kidney problems where the blood supply to your kidney is reduced (renal artery stenosis)
• During the last 6 months of pregnancy (see section below on “Pregnancy and breast-feeding”)
• If your blood pressure is abnormally low or unstable. Your doctor will need to make this assessment.
• If you have diabetes or impaired kidney function and you are treated with a blood pressure lowering medicine containing aliskiren
Do not take ramipril if any of the above apply to you. If you are not sure, talk to your
doctor before taking ramipril.
Warnings and precautions
Talk to your doctor or pharmacist before taking Ramipril Capsules
• You have problems with your heart, kidneys and/or liver.
• If you have collagen vascular disease such as scleroderma or systemic lupus erythematosus
• If you have lost a lot of body salts or fluids (through being sick (vomiting), having diarrhoea, sweating more than usual, being on a low salt diet, taking diuretics (water tablets) for a long time or having had dialysis)
• If you are going to have treatment to reduce your allergy to bee or wasp stings (desensitization)
• If you are going to receive an anaesthetic. This may be given for an operation or any dental work. You may need to stop your ramipril treatment one day beforehand; ask your doctor for advice
• If you have high amounts of potassium in your blood (shown in blood test results)
• You must tell your doctor if you think you are (or might become) pregnant. Ramipril capsules are not recommended in the first 3 months of pregnancy and may cause serious harm to your baby after 3 months of pregnancy, see section “Pregnancy and breast feeding”.
• If you are taking any of the following medicines used to treat high blood pressure:
- an angiotensin II receptor blocker (ARBs) (also known as sartans - for example valsartan, telmisartan, irbesartan), in particular if you have diabetes-related kidney problems
- aliskiren
Your doctor may check your kidney function, blood pressure, and the amount of electrolytes (e.g. potassium) in your blood at regular intervals.
See also information under the heading “Do not take Ramipril Capsules”
Children and adolescents
Ramipril capsules are not recommended for use in children and adolescents below 18 years of age because safety and efficacy of ramipril in children has not yet been established..
If any of the above apply to you (or you are not sure), talk to your doctor before taking Ramipril capsules
Other medicines and Ramipril Capsule
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines
This is because Ramipril Capsules can affect the way some other medicines work. Also some medicines can affect the way ramipril capsules works.
Please tell your doctor if you are taking any of the following medicines. They can make ramipril capsules work less well:
• Medicines used to relieve pain and inflammation (e.g. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) such as ibuprofen or indometacin and aspirin)
• Medicines used for the treatment of low blood pressure, shock, cardiac failure, asthma or allergies such as ephedrine, noradrenaline or adrenaline. Your doctor will need to check your blood pressure.
Please tell your doctor if you are taking any of the following medicines. They can increase the chance of getting side effects if you take them with ramipril capsules:
Medicines used to relieve pain and inflammation (e.g. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) such as ibuprofen or indometacin and aspirin)
• Medicines for cancer (chemotherapy)
• Medicines to stop the rejection of organs after a transplant such as cyclosporine
• Diuretics (water tablets) such as furosemide
• Medicines which can increase the amount of potassium in your blood such as spironolactone, triamterene, amiloride, potassium salts and heparin (for thinning blood)
• Steroid medicines for inflammation such as prednisolone
• Allopurinol (used to lower the uric acid in your blood)
• Procainamide (for heart rhythm problems).
Please tell your doctor if you are taking any of the following medicines. They may be affected by ramipril:
• Medicines for diabetes such as oral glucose lowering medicines and insulin. Ramipril may lower your blood sugar amounts. Check your blood sugar amounts closely while taking ramipril
• Lithium (for mental health problems). Ramipril may increase the amount of lithium in your blood. Your lithium amount will need to be closely checked by your doctor.
If any of the above apply to you (or you are not sure), talk to your doctor before taking ramipril.
Your doctor may need to change your dose and/or to take other precautions:
If you are taking an angiotensin II receptor blocker (ARB) or aliskiren (see also information under the headings “Do not take Ramipril Capsules” and “Warnings and precautions”)
Ramipril capsules with food and drink
Ramipril Capsules may be taken with or without food.
Drinking alcohol with ramipril may make you feel dizzy or light-headed. If you are concerned about how much you can drink while you are taking ramipril, discuss this with your doctor as medicines used to reduce blood pressure and alcohol can have additive effects.
Pregnancy and breast-feeding
You must tell your doctor if you think that you are (or might become) pregnant.
You should not take ramipril in the first 12 weeks of pregnancy, and you must not take them at all after the 13th week as their use during pregnancy may possibly be harmful to the baby.
If you become pregnant while on ramipril, tell your doctor immediately. A switch to a suitable alternative treatment should be carried out in advance of a planned pregnancy.
You should not take ramipril if you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
You may feel dizzy, while taking ramipril. This is more likely to happen when you start taking ramipril or start taking a higher dose. If this happens, do not drive or use any tools or machines.
Ramipril Capsules contain ponceau 4R (E124) & Sunset yellow (E 110)
Your medicine contains a small amount of an inactive ingredient called ponceau 4R (E124) & Sunset yellow (E 110) that may cause allergic reactions.
3. How to take Ramipril Capsules
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Swallow the capsule(s) whole with a glass of water. The capsules can be taken before, with or after food. To help you remember to take your medicine, try to get into the habit of taking it at the same time each day. Do not crush or chew the capsules.
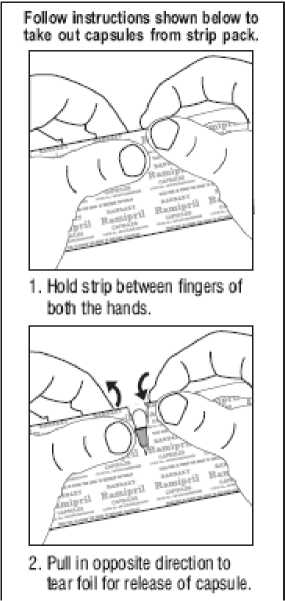
How much to take
Treatment of high blood pressure
• The usual starting dose is 1.25 mg or 2.5 mg once daily.
• Your doctor will adjust the amount you take until your blood pressure is controlled.
• The maximum daily dose should not exceed 10 mg.
• If you are taking water tablets (diuretics) already, your doctor may stop or reduce the amount of the diuretic you take before beginning treatment with ramipril
To reduce the risk of you having a heart attack or stroke
• The usual starting dose is 2.5 mg once daily.
• Your doctor may then decide to increase the amount you take.
• The usual dose is 10 mg once daily.
Treatment to reduce or delay the worsening of kidney problems
• You may be started on a dose of 1.25 mg or 2.5 mg once daily.
• Your doctor will adjust the amount you are taking.
• The usual dose is 5 mg or 10 mg once daily.
Treatment of heart failure
• The usual starting dose is 1.25 mg once daily.
• Your doctor will adjust the amount you take.
• The maximum dose is 10 mg daily. Two administrations per day are preferable.
Treatment after you have had a heart attack
• The usual starting dose is 1.25 mg once daily to 2.5 mg twice daily.
• Your doctor will adjust the amount you take.
• The usual dose is 10 mg daily. Two administrations per day are preferable.
Older people
Your doctor will reduce the initial dose and adjust your treatment more slowly.
If you forget to take Ramipril Capsules at the right time, take them as soon as you remember. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not take a double dose to make up for a forgotten capsule.
If you take more Ramipril Capsules than you should, consult your doctor or go to the nearest hospital casualty department immediately. Do not drive to the hospital, get somebody else to take you or call for an ambulance. Take this leaflet or the medicine pack with you so your doctor will know what you have taken.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets
them.
Stop taking ramipril and see a doctor straight away, if you notice any of the
following serious side effects - you may need urgent medical treatment:
• Swelling of the face, lips or throat which make it difficult to swallow or breathe, as well as itching and rashes. This could be a sign of a severe allergic reaction to ramipril
• Severe skin reactions including rash, ulcers in your mouth, worsening of a preexisting skin disease, reddening, blistering or detachment of skin (such as Stevens-Johnson syndrome, toxic epidermal necrolysis or erythema multiform).
Tell your doctor immediately if you experience:
• Faster heart rate, uneven or forceful heartbeat (palpitations), chest pain, tightness in your chest or more serious problems including heart attack and stroke
• Shortness of breath or a cough. These could be signs of lung problems
• Bruising more easily, bleeding for longer than normal, any sign of bleeding (e.g. bleeding from the gums), purple spots blotching on the skin or getting infections more easily than usual, sore throat and fever, feeling tired, faint, dizzy or having pale skin. These can be signs of blood or bone marrow problems
• Severe stomach pain which may reach through to your back. This could be a sign of pancreatitis (inflammation of the pancreas).
• Fever, chills, tiredness, loss of appetite, stomach pain, feeling sick, yellowing of your skin or eyes (jaundice). These can be signs of liver problems such as hepatitis (inflammation of the liver) or liver damage.
Other side effects include:
Please tell your doctor if any of the following gets serious or lasts longer than a few days.
Common (affects up to 1 in 10 people)
• Headache or feeling tired
• Feeling dizzy. This is more likely to happen when you start taking ramipril or start taking a higher dose
• Fainting, hypotension (abnormally low blood pressure), especially when you stand or sit up quickly
• Dry tickly cough, inflammation of your sinuses (sinusitis) or bronchitis, shortness of breath
• Stomach or gut pain, diarrhoea, indigestion, feeling or being sick
• Skin rash with or without raised area
• Chest pain
• Cramps or pain in your muscles
• Blood tests showing more potassium than usual in your blood.
Uncommon (affects up to 1 in 100 people)
• Balance problems (vertigo)
• Itching and unusual skin sensations such as numbness, tingling, pricking, burning or creeping on your skin (paraesthesia)
• Loss or change in the way things taste
• Sleep problems
• Feeling depressed, anxious, more nervous than usual or restless
• Blocked nose, difficulty breathing or worsening of asthma
• A swelling in your gut called “intestinal angioedema” presenting with symptoms like abdominal pain, vomiting and diarrhoea
• Heartburn, constipation or dry mouth
• Passing more water (urine) than usual over the day
• Sweating more than usual
• Loss or decrease of appetite (anorexia)
• Increased or irregular heartbeats, swollen arms and legs. This may be a sign of your body holding onto more water than usual
• Flushing
• Blurred vision
• Pain in your joints
• Fever
• Sexual inability in men, reduced sexual desire in men or women
• An increased number of certain white blood cells (eosinophilia) found during a blood test
• Blood tests showing change in the way your liver, pancreas or kidneys are working.
Rare (affects up to 1 in 1,000 people)
• Feeling shaky or confused
• Red and swollen tongue
• Severe flaking or peeling of the skin, itchy, lumpy rash
• Nail problem (e.g. loosening or separation of a nail from its bed)
• Skin rash or bruising
• Blotches on your skin and cold extremities
• Red, itchy, swollen or watery eyes
• Disturbed hearing and ringing in your ears
• Feeling weak
• Blood tests showing a decrease in the number of red blood cells, white blood cells or platelets or in the amount of haemoglobin.
Very rare (affects up to 1 in 10,000 people)
• Being more sensitive to the sun than usual.
Other side effects reported:
Please tell your doctor if any of the following gets serious or lasts longer than a few days.
• Difficulty concentrating
• Swollen mouth
• Blood tests showing too few blood cells in your blood
• Blood tests showing less sodium than usual in your blood
• Fingers and toes changing colour when you are cold and then tingling or feeling painful when you warm up (Raynaud's phenomenon)
• Breast enlargement in men
• Slowed or impaired reactions
• Burning sensation
• Change in the way things smell
• Hair loss.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine
5. How to store Ramipril Capsules
Keep this medicine out of the sight and reach of children.
Do not take Ramipril Capsules after the expiry date which is stated on the carton (EXP). The expiry date refers to the last day of that month.
Store in the original package.
Do now throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment
6. Contents of the pack and other information What Ramipril capsules contain
The active ingredient is ramipril. Each capsule contains 2.5 mg ramipril
The other ingredients are pregelatinised starch (Maize), gelatin, titanium dioxide (E171),
sunset yellow (E110) and ponceau 4R (E124).
The black printing ink contains shellac, propylene glycol, potassium hydroxide and black iron oxide (E172).
What Ramipril capsules look like and contents of the pack
Ramipril 2.5 mg Capsules are orange and white printed with ‘R’ on the cap and ‘2.5’ on the body. They contain a white to off-white granular powder.
Ramipril capsules are available as strip packs of 21, 28, 30, 56 and 60 capsules. Not all pack sizes may be marketed.
Marketing Authorisation Holder:
Ranbaxy (UK) Limited Building 4, Chiswick Park 566 Chiswick High Road London, W4 5YE United Kingdom
Manufacturer:
Ranbaxy Ireland Limited Spafield, Cork Road,
Cashel, Co-Tipperary Ireland
Basics GmBH, 201 Hemmelrather Weg, Gebaude Giz 1,
Leverkusen 51377, Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
UK: Ramipril 2.5 mg Capsules, hard PL: Ramicor capsules 2.5 mg IE: Bellramil Capsules 2.5 mg, hard
This leaflet was last revised in October 2014.