Recivit 533 Mcg Sublingual Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Recivit 533 mcg Sublingual Tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Recivit 533 microgram sublingual tablets:
Each tablet contains 840 micrograms of fentanyl citrate, equivalent to 533 micrograms of fentanyl.
Excipient with known effect:
Each tablet contains 0.651 mg sodium.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Sublingual tablet
Recivit 533 microgram sublingual tablets:
This medicine is presented in the form of a white, convex, triangular tablet, height of 5.6 mm, printed with ‘5’ in black ink on one face.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Recivit is indicated for the treatment of breakthrough pain (BTP) in adults with cancer who are already receiving maintenance opioid therapy for chronic cancer pain.
BTP is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain.
Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
4.2 Posology and method of administration
Treatment should be initiated by and remain under the guidance of a physician experienced in the management of opioid therapy in cancer patients. Physicians should keep in mind the potential of abuse of fentanyl. Patients should be instructed not to use two different formulations of fentanyl concurrently for the treatment of breakthrough pain, and to dispose of any fentanyl product prescribed for BTP when switching to Recivit. The number of tablet strengths available to the patients at any time should be minimised to prevent confusion and potential overdose.
Recivit should be administered directly under the tongue at the deepest part.
Recivit should not be swallowed, but allowed to completely dissolve in the sublingual cavity without chewing or sucking. Patients should be advised not to eat or drink anything until the sublingual tablet is completely dissolved.
After 30 minutes, if remnants from the Recivit tablet remain, they may be swallowed.
In patients who have a dry mouth, water may be used to moisten the buccal mucosa before taking Recivit.
The tablet should not be stored once removed from the blister package as the tablet integrity cannot be guaranteed and a risk of accidental exposure to a tablet can occur (see also section 4.4 for warnings in children).
Patients should be advised to keep Recivit in a locked storage space.
Dose Titration
Before patients are titrated with Recivit, it is expected that their background persistent pain will be controlled by use of opioid therapy and that they are typically experiencing no more than 4 episodes of breakthrough pain per day.
The object of dose titration is to identify an optimal maintenance dose for ongoing treatment of breakthrough pain episodes. This optimal dose should provide adequate analgesia with an acceptable level of adverse reactions.
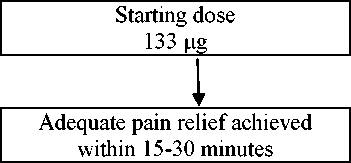
The optimal dose of Recivit will be determined by upward titration, on an individual patient basis. Several doses are available for use during the dose titration phase. The initial dose of Recivit used should be 133 micrograms, titrating upwards as necessary through the range of available dosage strengths.
Patients should be carefully monitored until an optimal dose is reached.
Switching from other fentanyl containing products to Recivit must not occur at a 1:1 ratio because of different absorption profiles. If patients are switched from another fentanyl containing product, a new dose titration with Recivit is required.
The following dose regimen is recommended for titration, although in all cases the physician should take into account the clinical need of the patient, age and concomitant illness.
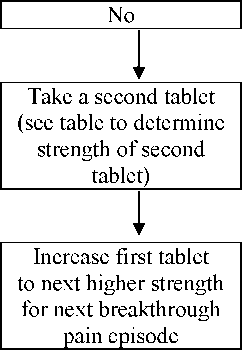
All patients must start therapy with a single 133 micrograms sublingual tablet. If adequate analgesia is not obtained within 15-30 minutes of administration of a single tablet, a supplemental (second) 133 micrograms tablet may be administered. If treatment of a breakthrough pain episode requires more than one dosage unit, an increase in dose to the next higher available strength should be considered (Refer to figure below). Dose escalation should continue in a stepwise manner until adequate analgesia is achieved. The dose strength for the supplemental (second) tablet should be increased from 133 to 267 micrograms at doses of 533 micrograms. This is illustrated in the schedule below. No more than two (2) tablets should be administered for a single episode of breakthrough pain during this titration phase.
RECIVIT TITRATION PROCESS
Yes
Use this dose for Subsequent breakthrough pain episodes
|
Strength (micrograms) of first tablet per episode of breakthrough pain |
Strength (micrograms) of supplemental (second) tablet to be taken 15-30 minutes after first tablet, if required |
|
133 |
133 |
|
267 |
133 |
|
400 |
133 |
|
533 |
267 |
|
800 |
- |
If adequate analgesia is achieved at the higher dose, but undesirable effects are considered unacceptable, an intermediate dose may be administered (using the 67 micrograms or 133 micrograms tablet).
Doses higher than 800 micrograms have not been evaluated in clinical studies.
In order to minimise the risk of opioid-related adverse reactions and to identify the appropriate dose, it is imperative that patients be monitored closely by health professionals during the titration process.
Maintenance therapy
Once an appropriate dose has been established, which may be more than one tablet, patients should be maintained on this dose and should limit consumption to a maximum of four Recivit doses per day.
Dose re-adjustment
If the response (analgesia or adverse reactions) to the titrated Recivit dose markedly changes, an adjustment of dose may be necessary to ensure that an optimal dose is maintained.
If more than four episodes of breakthrough pain are consistently experienced per day, then the dose of the long acting opioid used for persistent pain should be re-evaluated. If the long acting opioid or dose of long acting opioid is changed, the Recivit dose should be re-evaluated and re-titrated as necessary to ensure the patient is on an optimal dose.
It is imperative that any dose re-titration of any analgesic is monitored by a health professional.
Discontinuation of therapy
Recivit should be immediately discontinued if no longer required. For patients requiring discontinuation of all opioid therapy, account should be taken of the Recivit dose in consideration of a gradual downward opioid titration to avoid the possibility of abrupt withdrawal effects.
Use in elderly patients
Dose titration needs to be approached with particular care and patients observed carefully for signs of fentanyl toxicity (see section 4.4).
Use in patients with renal and hepatic impairment
Patients with kidney or liver dysfunction should be carefully observed for signs of fentanyl toxicity during the Recivit titration phase (see section 4.4).
Use in paediatric population
Recivit is not indicated for use in children and adolescents below 18 years due to a lack of data on safety and efficacy (see also section 4.4).
4.3 Contraindications
• Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
• Patients without maintenance opioid therapy (see section 4.1) as there is an increased risk of respiratory depression.
• Simultaneous use of monoamine-oxidase (MAO) inhibitors, or within 2 weeks after the cessation of the use of MAO inhibitors.
• Severe respiratory depression or severe obstructive lung conditions.
• Treatment of acute pain other than breakthrough pain.
4.4 Special warnings and precautions for use
Patients and their carers must be instructed that Recivit contains an active substance in an amount that can be fatal to a child, and therefore to keep all tablets out of the reach and sight of children and non-patients at all times.
In order to minimise the risks of opioid-related undesirable effects and to identify the effective dose, it is imperative that patients be monitored closely by health professionals during the titration process.
It is important that the long acting opioid treatment used to treat the patient’s persistent pain has been stabilised before Recivit therapy begins and that the patient continues to be treated with the long acting opioid treatment whilst taking Recivit.
As with all opioids, there is a risk of clinically significant respiratory depression associated with the use of fentanyl. Particular caution should be used when titrating Recivit in patients with non-severe chronic obstructive pulmonary disease or other medical conditions predisposing them to respiratory depression, as even normally therapeutic doses of Recivit may further decrease respiratory drive to the point of respiratory failure.
Recivit should only be administered with extreme caution in patients who may be particularly susceptible to the intracranial effects of CO2 retention, such as those with evidence of increased intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of a patient with a head injury and should be used only if clinically warranted.
Cardiac disease
Fentanyl may produce bradycardia. Fentanyl should be used with caution in patients with previous or preexisting bradyarrhythmias.
In addition, Recivit should be administered with caution to patients with hepatic or renal impairment. The influence of hepatic and renal impairment on the pharmacokinetics of the medicinal product has not been evaluated, however, when administered intravenously the clearance of fentanyl has been shown to be altered in hepatic and renal impairment due to alterations in metabolic clearance and plasma proteins. After administration of Recivit, impaired hepatic and renal function may both increase the bioavailability of swallowed fentanyl and decrease its systemic clearance, which could lead to increased and prolonged opioid effects. Therefore, special care should be taken during the titration process in patients with moderate or severe hepatic or renal impairment.
Careful consideration should be given to patients with hypovolaemia and hypotension.
Recivit has not been studied in patients with mouth wounds or mucositis. There may be a risk of increased systemic drug exposure in such patients and therefore extra caution is recommended during dose titration.
Tolerance and physical and/or psychological dependence may develop upon repeated administration of opioids such as fentanyl. However, iatrogenic addiction following therapeutic use of opioids is rare.
Serotonin Syndrome
Caution is advised when Recivit is coadministered with drugs that affect the serotoninergic neurotransmitter systems.
The development of a potentially life-threatening serotonin syndrome may occur with the concomitant use of serotonergic drugs such as Selective Serotonin Re-uptake Inhibitors (SSRIs) and Serotonin Norepinephrine Re-uptake Inhibitors (SNRIs), and with drugs which impair metabolism of serotonin (including Monoamine Oxidase Inhibitors [MAOIs]). This may occur within the recommended dose.
Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhoea).
If serotonin syndrome is suspected, treatment with Recivit should be discontinued.
This medicinal product contains 0.651 mg sodium per tablet. To be taken into consideration by patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
Serotoninergic Drugs
Coadministration of fentanyl with a serotoninergic agent, such as a Selective Serotonin Re-uptake Inhibitor (SSRI) or a Serotonin Norepinephrine Re-uptake Inhibitor (SNRI) or a Monoamine Oxidase Inhibitor (MAOI), may increase the risk of serotonin syndrome, a potentially life-threatening condition.
Recivit is contraindicated for use in patients who have received monoamine oxidase (MAO) inhibitors within 14 days because severe and unpredictable potentiation by MAO inhibitors has been reported with opioid analgesics.
Fentanyl is metabolized by the CYP3A4 isoenzyme in the liver and intestinal mucosa. Inhibitors of CYP3A4 such as
• macrolide antibiotics (e.g. erythromycin, clarithromycin, telithromycin),
• azole antifungals (e.g. ketoconazole, itraconazole, and fluconazole),
• certain protease inhibitors (e.g. ritonavir, indinavir, nelfinavir, saquinavir),
• calcium channel blockers (e.g. diltiazem or verapamil),
• anti-emetics (e.g. aprepitant or dronabinol),
• antidepressants (e.g. fluoxetin),
• antacids (e.g. cimetidin)
or alcohol may increase the bioavailability of swallowed fentanyl and may also decrease its systemic clearance which may result in increased or prolonged opioid effects and may cause potentially fatal respiratory depression. Similar effects could be seen after concurrent ingestion of grapefruit juice, which is known to inhibit CYP3A4. Hence caution is advised if fentanyl is given concomitantly with CYP3A4 inhibitors. Patients receiving Recivit who begin therapy with, or increase the dose of, CYP3A4 inhibitors should be carefully monitored for signs of opioid toxicity over an extended period of time.
The concomitant use of Recivit with potent CYP3A4 inducers such as
• barbiturates and other sedatives (e.g. phenobarbital),
• anti-epileptics (e.g. carbamazepine, phenytoin, oxcarbazepine),
• certain antivirals (e.g. efavirenz, nevirapine),
• anti-inflammatory or immunosuppressive agents (e.g. glucocorticoids),
• anti-diabetics (e.g. pioglitazone),
• antibiotics for tuberculosis treatment (e.g. rifabutin, rifampin),
• psychotropic substances (e.g. modafinil),
• antidepressants (e.g. St. John's wort),
may result in a decrease in fentanyl plasma concentrations, which could decrease the efficacy of Recivit. Patients receiving Recivit who stop therapy with, or decrease the dose of, CYP3A4 inducers should be monitored for signs of increased Recivit activity, or toxicity, and the dose of Recivit should be adjusted accordingly.
The concomitant use of other central nervous system depressants, including other opioids, sedatives or hypnotics, general anaesthetics, phenothiazines, tranquillisers, skeletal muscle relaxants, sedating antihistamines and alcohol may produce additive depressant effects.
The concomitant use of partial opioid agonists/antagonists (e.g. buprenorphine, nalbuphine, pentazocine) is not recommended. They have high affinity to opioid receptors with relatively low intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce withdrawal symptoms in opioid dependant patients.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Recivit should not be used in pregnancy unless clearly necessary.
Following long-term treatment, fentanyl may cause withdrawal in the new-born infant.
It is advised not to use fentanyl during labour and delivery (including caesarean section) because fentanyl passes through the placenta and may cause respiratory depression in the foetus or in the new-born infant. If Recivit is administered, an antidote for the child should be readily available.
Breastfeeding
Fentanyl passes into breast milk and may cause sedation and respiratory depression in the breast-fed child. Fentanyl should not be used by breastfeeding women and breastfeeding should not be restarted until at least 48 hours after the last administration of fentanyl.
4.7 Effects on ability to drive and use machines
No studies of the effects on the ability to drive and use machines have been performed.
However, opioid analgesics impair the mental and/or physical ability required for the performance of potentially dangerous tasks (e.g., driving a car or operating machinery). Patients should be advised not to drive or operate machinery if they experience somnolence, dizziness, or visual disturbance while taking Recivit and not to drive or operate machinery until they know how they react.
4.8 Undesirable effects
Typical opioid side effects are to be expected with Recivit. Frequently, these will cease or decrease in intensity with continued use of the product, as the patient is titrated to the most appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients should be closely monitored for these.
The most frequently observed adverse reactions include: nausea, vomiting, constipation, headache, somnolence/fatigue and dizziness.
The following adverse reactions have been reported with Recivit and/or other fentanyl-containing compounds during clinical studies and post marketing experience in real-world drug practice with concomitant opioid treatment. Thus it is not possible to definitively separate the effects of fentanyl alone.
Adverse reactions are listed below as MedDRA preferred term by system organ class and frequency (frequencies are defined as: very common > 1/10, common > 1/100 to <1/10, uncommon > 1/1,000 to <1/100, rare >1/10,000 to <1/1,000, very rare <1/10,000), not known (cannot be estimated from the available data):
|
MedDRA system organ class |
Very common |
Common |
Uncommon |
Not known |
|
Metabolism and nutrition disorders |
anorexia | |||
|
Psychiatric disorders |
confusion, anxiety, hallucinations, abnormal thinking |
abnormal dreams, depersonalisatio , depression, emotional lability, euphoria | ||
|
Nervous system disorders |
somnolence, sedation, dizziness |
loss of consciousness, vertigo, headache, myoclonus, taste perversion |
coma, convulsion, paraesthesia (including hyperaesthesia/ circumoral paraesthesia), abnormal gait/ incoordination |
|
Eye disorders |
abnormal vision (blurred, double vision) | |||
|
Vascular disorders |
hypotension |
flushing and hot flush | ||
|
Respiratory, thoracic and mediastinal disorders |
dyspnoea, respiratory depression | |||
|
Gastrointesti nal disorders |
nausea, constipation |
vomiting, dry mouth, abdominal pain, dyspepsia, |
ileus, flatulence, abdomen enlarged, dental caries |
diarrhoea, tooth loss, gingival recession |
|
Skin and subcutaneous tissue disorders |
pruritus, sweating |
rash | ||
|
Renal and urinary disorders |
urinary retention | |||
|
General disorders and administratio n site conditions |
asthenia |
malaise |
fatigue, peripheral oedema | |
|
Injury, poisoning and procedural complications |
accidental injury (for example, falls) |
At the cessation of treatment, possible symptoms of withdrawal can be noticed, such as, anxiety, tremor, sweating, irritability, nausea and vomiting, diarrhoea.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
The symptoms of fentanyl overdosage are expected to be similar in nature to those of intravenous fentanyl and other opioids, and are an extension of its pharmacological actions, with the most serious significant effects being altered mental status, loss of consciousness, coma, cardiorespiratory arrest, respiratory depression, respiratory distress and respiratory failure, which have resulted in death.
Immediate management of opioid overdose includes removal of the Recivit if still in the mouth, ensuring a patent airway, physical and verbal stimulation of the patient, assessment of the level of consciousness, ventilatory and circulatory status, and assisted ventilation (ventilatory support) if necessary.
For treatment of overdosage (accidental ingestion) in the opioid naive person, intravenous access should be obtained, and naloxone or other opioid antagonists should be employed as clinically indicated. The duration of respiratory depression following overdose may be longer than the effects of the opioid antagonist's action (e.g., the half-life of naloxone ranges from 30 to 81 minutes) and repeated administration may be necessary. Consult the Summary of Product Characteristics of the individual opioid antagonist for details about such use.
For treatment of overdose in opioid-maintained patients, intravenous access should be obtained. The judicious use of naloxone or another opioid antagonist may be warranted in some instances, but it is associated with the risk of precipitating an acute withdrawal syndrome.
If severe or persistent hypotension occurs, hypovolaemia should be considered, and the condition should be managed with appropriate parenteral fluid therapy.
Muscle rigidity interfering with respiration has been reported with fentanyl and other opioids. In this situation, endotracheal intubation, assisted ventilation and administration of opioid antagonists as well as muscle relaxants may be requested.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: phenylpiperidine derivatives.
ATC code: N02AB03.
Fentanyl is an opioid analgesic, interacting predominantly with the opioid p-receptor. Its primary therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory depression, bradycardia, hypothermia, constipation, myosis, physical dependence and euphoria.
The analgesic effects of fentanyl are related to its plasma level. In general, the effective concentration and the concentration at which toxicity occurs increase with increasing tolerance to opioids. The rate of development of tolerance varies widely among individuals. As a result, the dose of Recivit should be individually titrated to achieve the desired effect (see section 4.2).
The efficacy and safety of Recivit have been assessed in a double blind randomized, placebo-controlled, cross over study in 91 opioid-treated adult cancer patients who experienced 1 to 4 episodes of breakthrough pain (BTP) per day. The primary endpoint was the sum of pain intensity difference at 30 minutes (SPID30) after dosing which was statistically significant compared to placebo (p< 0.0001).
Sum of pain intensity difference from 6 minutes after dosing and up to 60 minutes was also significant compared to placebo (respectively p=0.02 after 6 minutes and p<0.0001 after 60 minutes).
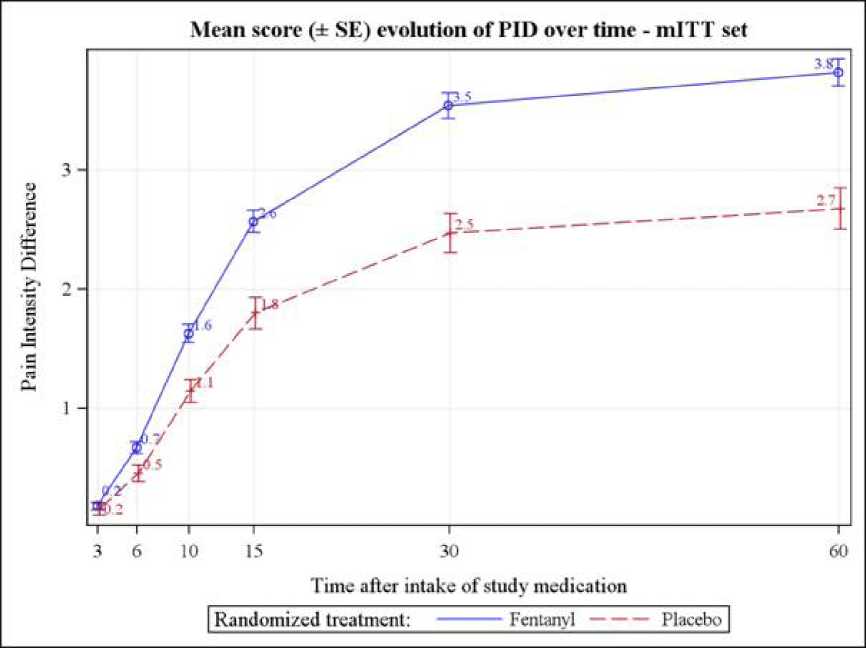
The secondary outcome measure mean pain intensity difference (PID) was significantly higher for the BTP episodes treated with fentanyl than those treated with placebo from 6 minutes after dosing and up to 60 minutes (respectively p=0.003 and p<0.0001) (see figure below).
Also, the mean pain relief was significantly higher for the BTP episodes treated with fentanyl than those treated with placebo from 6 minutes after dosing and up to 60 minutes. The difference in mean pain relief between active treatment and placebo was 0.1 (on a 5-point Numerical Rating Scale) at 6 minutes (p=0.002), 0.4 at 10 minutes, 0.5 at 15 minutes, 0.7 at 30 minutes and 0.7 at 60 minutes (p<0.0001).
All opioid p-receptor agonists, including fentanyl, produce dose dependent respiratory depression. The risk of respiratory depression is less in patients receiving chronic opioid therapy as these patients will develop tolerance to respiratory depressant effects.
While opioids generally increase the tone of urinary tract smooth muscle, the net effect tends to be variable, in some cases producing urinary urgency, in others, difficulty in urination.
Opioids increase the tone and decrease the propulsive contractions of the smooth muscle of the gastrointestinal tract leading to a prolongation in gastrointestinal transit time, which may be responsible for the constipating effect of fentanyl.
5.2 Pharmacokinetic properties
Fentanyl is highly lipophilic and can be absorbed very rapidly through the oral mucosa and more slowly through the gastrointestinal tract. Orally administered fentanyl undergoes pronounced hepatic and intestinal first pass effects and the metabolites do not contribute to fentanyl’s therapeutic effects.
Recivit employs a technology that allows rapid release of fentanyl and enhances the rate and extent of fentanyl absorbed through the oral mucosa. The absolute bioavailability of Recivit has not been determined but is estimated to be about 70%.
Absorption
Mean maximal plasma concentrations range from 360 to 2070 pg/ml (after administration of 133 to 800 pg of Recivit) and are reached within 50 and 90 minutes.
Distribution
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent
volume of distribution. After sublingual administration of Recivit, fentanyl undergoes initial rapid distribution that represents an equilibration of fentanyl between plasma and the highly perfused tissues (brain, heart and lungs). Subsequently, fentanyl is redistributed between the deep tissue compartment (muscle and fat) and the plasma. The plasma protein binding of fentanyl is 80% to 85%. The main binding protein is alpha-1-acid
glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of fentanyl increases with acidosis.
Biotransformation and elimination
Fentanyl is metabolised in the liver and in the intestinal mucosa to norfentanyl by CYP3A4 isoform. Norfentanyl is not pharmacologically active in animal studies. More than 90% of the administered dose of fentanyl is eliminated by biotransformation to N-dealkylated and hydroxylated inactive metabolites.
Following the intravenous administration of fentanyl, less than 7% of the administered dose is excreted unchanged in the urine, and only about 1% is excreted unchanged in the faeces. The metabolites are mainly excreted in the urine, while faecal excretion is less important.
The terminal elimination phase of fentanyl is the result of the redistribution between plasma and a deep tissue compartment. Following the administration of Recivit, the terminal elimination half-life is approximately 12 hours.
Linearity/non linearity
Dose proportionality from 133 micrograms to 800 micrograms has been demonstrated.
Renal/hepatic impairment
Impaired hepatic or renal function could cause increased serum concentrations. Elderly, cachectic or generally impaired patients may have a lower fentanyl clearance, which could cause a longer terminal half-life for the compound (see sections 4.2 and 4.4).
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety
pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound induced malformations or developmental variations when administered during the period of organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1 pups were delayed physical development, sensory functions, reflexes and behaviour. These effects could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year subcutaneous carcinogenicity study in rats) with fentanyl did not reveal any findings indicative of oncogenic potential. Evaluation of brain slides from the carcinogenicity study in rats revealed brain lesions in animals administered high doses of fentanyl citrate. The relevance of these findings to humans is unknown.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Calcium hydrogen phosphate anhydrous Microcrystalline cellulose Disodium phosphate anhydrous Hypromellose Macrogol
Magnesium stearate
Maltodextrin
Titanium dioxide (E171)
Triacetin
Printing ink [shellac, black iron oxide (E172)]
6.2 Incompatibilities
Not applicable
6.3 Shelf life
3 years
6.4 Special precautions for storage
This medicinal product does not require any special temperature storage conditions. Store in the original blister package, in order to protect from light.
6.5 Nature and contents of container
Peelable, child resistant blister:
- Polyamide-Aluminium-PVC / Aluminium foil blister, contained in a cardboard outer carton.
- Polyamide-Aluminium-PVC / Aluminium-PET foil blister, contained in a cardboard outer carton.
Pack sizes: 3, 4, 15 or 30 sublingual tablets.
Not all pack sizes may be marketed
6.6 Special precautions for disposal
Sublingual tablets with remaining active substance must not be disposed of in household waste.
Waste material should be disposed of safely. Patients/carers should be encouraged to dispose any unused product in accordance with national and local requirements.
7 MARKETING AUTHORISATION HOLDER
Grunenthal Ltd Regus Lakeside House 1 Furzeground Way Stockley Park East Uxbridge
Middlesex UB11 1BD United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 21727/0061
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
02/04/2013
10 DATE OF REVISION OF THE TEXT
25/06/2014