Regiocit Solution For Haemofiltration
UK IE MT
Package leaflet: Information for the user
Regiocit
Solution for haemofiltration
Citrate, Sodium, Chloride
Read all of this leaflet carefully
before you start using this
medicine because it contains
important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
WHAT IS IN THIS LEAFLET:
1. What this medicine is and what it is used for
2. What you need to know before you use this medicine
3. How to use this medicine
4. Possible side effects
5. How to store this medicine
6. Contents of the pack and other information
1. WHAT THIS MEDICINE IS AND WHAT IT IS USED FOR
This medicine is a solution for haemofiltration and prevents blood clotting during continuous renal replacement therapy (CRRT), which is a form of dialysis treatment. This medicine is used for critically ill patients when the normal medicine used to prevent blood clotting (heparin) is inappropriate. Citrate provides anticoagulation by binding to calcium in the blood.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE THIS MEDICINE 1 2
WARNING AND PRECAUTIONS
Talk to your doctor, pharmacist or nurse before using this medicine. This medicine is not for direct intravenous infusion. It should be used only with a machine capable of performing continuous renal replacement therapy (CRRT), which is a type of dialysis specifically for critically ill patients with kidney failure. The CRRT machine must be suitable for citrate anticoagulation.
If the overwrap or solution bag is damaged, the solution may become contaminated and must not be used. Besides this medicine, the treatment involves other fluids that are infused. The composition or rate of administration of these other fluids may need to be adjusted to be compatible with this medicine. Your doctor will closely monitor your haemodynamic status, fluid balance, glucose level, electrolyte and acid/base balance before and during treatment. Adjustments to the therapy will be made if needed. Special attention will be paid to the blood level of sodium, calcium and magnesium (electrolytes). Infusion of sodium may be needed to compensate for sodium loss. Infusion of calcium is standard practice. Infusion of magnesium may also be needed.
Your doctor will pay special attention to the citrate infusion rate. Too much citrate causes low blood levels of calcium and high blood pH, which may lead to neurologic and cardiac complications. High blood pH can be corrected by adjusting dialysis settings and by infusing 0.9% sodium chloride solution post-filter. Low blood levels of calcium can be treated by infusion of calcium.
Special attention is required by your doctor if you suffer from liver failure or shock. The metabolism of citrate may be markedly reduced resulting in accumulation of citrate acompanied by low blood pH. Your doctor will decide if your treatment has to be adjusted. If the total/ion-ized calcium ratio rises above 2.3, the citrate buffer should be reduced or stopped.
The instructions for use must be strictly followed. Incorrect use of the access ports or other restrictions to fluid flow might lead to incorrect patient weight loss and may result in machine alarms. Continuing treatment without resolving the originating cause may result in patient injury or death.
This medicine is for single use only. Any unused solution must be discarded.
Use only if the solution is clear and free from visible particles.
OTHER MEDICINES AND REGIOCIT
Tell your doctor, pharmacist or nurse if you are taking, or have recently taken, any other medicines, including medicines obtained without a prescription. This is because the concentration of other medicines may be reduced during dialysis treatment. Your doctor will decide if any changes in the dosage of your medicines should be made. In particular, tell your doctor if you are using medicinal product containing any of the following:
• Calcium; as they may increase the calcium level in your blood and/or
• Sodium hydrogen carbonate; as they may increase the hydrogen carbonate level in your blood.
PREGNANCY, BREAST-FEEDING AND FERTILITY
Fertility:
No effects on fertility are anticipated, since sodium, chloride and citrate are normal constituents of the body.
Pregnancy and breast-feeding: There are no documented clinical data on the use of this medicine during pregnancy and breastfeeding. This medicine should only be administered to pregnant and breast-feeding women if clearly needed.
DRIVING AND USING MACHINES
This medicine is not known to affect your ability to drive or use machines.
I 3 I
Regiocit D13000340 Rev. 2016-03 4798
3. HOW TO USE THIS
MEDICINE_
For intravenous use. This medicine is to be used in hospitals and administered by medical professionals only. The volume used, and therefore the dose of this medicine, will depend on your condition. The dose volume will be determined by your doctor.
The recommended flow rates for this medicine in adults and adolescents:
• In continuous veno-venous haemofiltration: 1 - 2.5 l/h with a blood flow rate between 100 and 200 ml/min.
• In continuous veno-venous haemodiafiltration: 1 - 2 l/h with a blood flow rate between 100 and 200 ml/min.
Use in older people:
The recommended flow rates are the same as for adults and adolescents. Use in children:
For neonates to toddlers (0 to 23 months) Regiocit should target a dose of 3 mmol citrate per litre of blood flow in continuous veno-venous haemofiltration or haemodiafiltration. For children (2 to 11 years) dosage should be adapted to both the weight of the patient and the blood flow rate.
Liver failure or shock:
In these conditions, the initial starting dose of citrate should be reduced.
INSTRUCTIONS FOR USE
Regiocit will be given to you in a hospital. Your doctor will know how to use Regiocit.
For instructions for use see the end of this leaflet.
4. POSSIBLE SIDE EFFECTS
NOT KNOWN: FREQUENCY CANNOT BE ESTIMATED FROM THE AVAILABLE DATA
• Imbalance in the level of fluid in the body (dehydration, retention of fluid in the body)
• Cramps2
• Side effects related to the dialysis treatment rather than this medicine.
REPORTING OF SIDE EFFECTS
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via
United Kingdom:
Via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
Republic of Ireland:
Via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2;
Tel: +353 1 6764971;
Fax: +353 1 6762517;
Website: www.hpra.ie E-mail: medsafety@hpra.ie
Malta:
Via The Medicines Authority, PostLicensing Directorate, 203, Level 3, Rue D'Argens, GZR 1368 Gzira Website:
Email:
postlicensing.medicinesauthority@
gov.mt
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE THIS
MEDICINE_
Keep this medicine out of the sight and reach of children.
This medicinal product does not require any special storage conditions.
Do not freeze.
Do not use this medicine after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
Do not use this medicine if you notice damage to the product or visible particles in the solution.
The solution can be disposed of via wastewater without harming the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
WHAT THIS MEDICINE CONTAINS
Composition:
Sodium chloride 5.03 g/l
Sodium citrate 5.29 g/l
The active substances are: Sodium, Na+ 140 mmol/l
Chloride, Cl- 86 mmol/l
Citrate, C6H5O73- 18 mmol/l
Theoretical osmolarity: 244 mOsm/l pH == 7.4
The other ingredients are:
• Dilute hydrochloric acid (for pH adjustment) E507
• Water for injections
WHAT THIS MEDICINE LOOKS LIKE AND CONTENTS OF THE PACK
This medicine is a clear and colourless solution for haemofiltation packed in a one-compartment bag made of a multilayer film containing polyolefins and elastomers.
The solution is sterile and free from bacterial endotoxins. Each bag contains 5000 ml solution and the bag is overwrapped with a transparent film. Each box contains two bags and one package leaflet.
MARKETING AUTHORISATION HOLDER
Gambro Lundia AB Magistratsvagen 16 226 43 Lund Sweden
MANUFACTURER
Gambro Dasco S.p.A.
Via Stelvio, 94 23035 Sondalo (SO)
Italy
This medicinal product is authorised in the Member States of the EEA under the following names: Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom: Regiocit
Bulgaria: Regiocit (Peruo^T)
This leaflet was last revised in 03/2016
I 4 |
Regiocit D13000340 Rev. 2016-03 4798
UK |E mT The following information is intended
UK I IE I Ml for healthcare professionals only
POSOLOGY_
The pre-filter infusion rate of Regiocit must be prescribed and adapted relative to the blood flow rate. The prescription of Regiocit must consider the flow rates of the effluent and other therapeutic fluids, the patient's fluid removal requirements, additional fluid inputs and outputs, and the desired acid-base and electrolyte balance.
Flow rate for anticoagulation of the extracorporeal circuit should be titrated to achieve a post-filter concentration of ionized calcium in the range 0.25 to 0.35 mmol/l. The patient's systemic ionized calcium concentration should be maintained in the normal physiologic range by adjustment of calcium supplementation.
Flow rates for Regiocit in adult and adolescents:
• In continuous veno-venous haemofiltration: 1 - 2.5 l/h with a blood flow rate between 100 and 200 ml/min.
• In continuous veno-venous haemodiafiltration: 1 - 2 l/h with a blood flow rate between 100 and 200 ml/min.
Paediatric population:
For neonates to toddlers (0 to 23 months) Regiocit should target a dose of 3 mmol citrate per litre of blood flow in continuous veno-venous haemofiltration or haemodiafiltration. For children (2 to 11 years) dosage should be
I 14 I
adapted to both the weight of the patient and the blood flow rate. Special populations:
In the elderly population there is no specific modification of the dosage compared to adults.
Hepatic impairment or shock:
In case of liver failure (including e.g. liver cirrhosis or acute liver failure) or shock, initial starting dose of citrate should be reduced as metabolism may be inadequate.
preparation
AND/OR HANDLING_
The solution can be disposed of via wastewater without harming the environment.
Remove the overwrap from the bag immediately before use.
Aseptic technique should be used throughout administration to the patient. The solution should be used immediately after opening to avoid microbiological contamination.
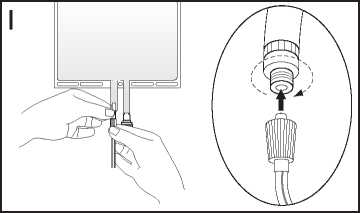
I If the luer connector is used, remove the cap with a twist and pull motion. Connect the male luer lock on the pre-blood pump line to the female luer connector on the bag using a push and twist motion. Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely.
(See figure I below.)
When the pre-blood pump line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer is a needle-less and swabbable port.
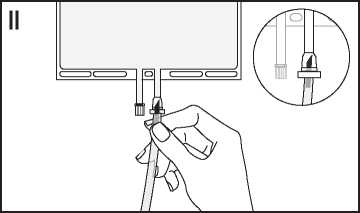
II If the injection connector (or spike connector) is used, remove the snap-off cap. Introduce the spike through the rubber septum. Verify that the fluid is flowing freely.
(See figure II below.)
X...............................................................................................................................................................................................................................................................................................................................X



Gambro and Regiocit are trademarks of Baxter International Inc., or its subsidiaries
y ° O E -Q CO CnJ -g
03 O LLJ 5 CD Cl (I) (I)
Q
DO NOT USE THIS MEDICINE IN
CASE OF:
• Allergy to the active substances or to any of the other ingredients (listed in section 6)
• Severely impaired liver function
Severely decreased blood flow in the muscles
Like all medicines, this medicine can cause side effects, although not everybody gets them. Your blood will be regularly controlled by a doctor or nurse in order to find possible side effects. Use of this solution could cause:
COMMON: MAY AFFECT UP TO 1 IN 10 PEOPLE
• Acid/base imbalance in the blood
Imbalances in the level of electrolytes in the blood (e.g. drop in the calcium, sodium, and/or magnesium level in the blood or increase in the calcium level in the blood)
OVERDOSE_
Undesirable administration of too high volumes of replacement solution may lead to an overdose, which can cause a life threatening situation for the patient. This may result in pulmonary oedema and congestive heart failure in relation with fluid overload and in hypoc-alcaemia and metabolic alkalosis due to citrate overload in relation to the blood flow. This derangement needs to be corrected immediately by stopping/lowering the amount of replacement solution and by the intravenous administration of calcium.
In patients with poor citrate metabolism (liver failure or shock) citrate may accumulate. Metabolic acidosis and ionized hypocalcae-mia may ensue. Regiocit should thus be either reduced or stopped.
To correct for metabolic acidosis, hydrogen carbonate has to be replaced. Continuous renal replacement therapy can be continued without anticoagulation or other means of anticoagulation have to be considered.
Regiocit D13000340 Rev. 2016-03 4798