Relevtec 52.5 Microgram/Hour Transdermal Patch
SZ00000LT000
PACKAGE LEAFLET: INFORMATION FOR THE USER
Relevtec 35 microgram/hour transdermal patch Relevtec 52.5 microgram/hour transdermal patch Relevtec 70 microgram/hour transdermal patch
Buprenorphine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
00000000
What is in this leaflet
1. What Relevtec is and what it is used for
2. What you need to know before you use Relevtec
3. How to use Relevtec
4. Possible side effects
5. How to store Relevtec
6. Contents of the pack and other information
IWhat Relevtec is and what it is used for
Relevtec contains the active substance buprenorphine which is a strong painkiller and belongs to a group of medicines called opioids. It acts on specific nerve cells in the spinal cord and in the brain.
Relevtec is used to treat
• moderate to severe cancer pain and severe pain that has not responded to other types of painkillers.
This medicine is not suitable for treating short-lasting pain.
2 What you need to know before you use Relevtec
Do not use Relevtec if you:
• are allergic to buprenorphine or any of the other ingredients of this medicine (listed in section 6)
• are dependent on opioids Relevtec must not be used to treat withdrawal symptoms of addicted persons.
• have serious breathing problems
• are being treated or have been treated during the last 2 weeks with medicines known as monoamine oxidase (MAO) inhibitors These types of medicines are used to treat depression or Parkinson's disease.
• have myasthenia gravis (a certain type of severe muscle weakness)
• have delirium tremens (confusion and trembling caused by abstinence from alcohol following habitual excessive drinking or occurring during an episode of heavy alcohol consumption)
Warnings and precautions
Talk to your doctor or pharmacist before using Relevtec if you:
• have recently drunk a lot of alcohol
• have convulsive disorders (fits)
• have unexplained loss of consciousness
• have a shock
• have increased pressure inside the skull such as after head injury
• have reduced breathing function
• are using medicines that reduce breathing listed under “Other medicines and Relevtec”
• have reduced liver function
Patients with reduced liver function require careful monitoring by the doctor during treatment.
• have fever or exposure to external heat Fever and external heat increase skin permeability, which may cause a higher buprenorphine blood level. Therefore, consult your doctor if you have fever. External heat - such as sauna, infrared lamp, electric blanket, hot water bottle - may prevent the transdermal patch from sticking properly. Do not expose yourself to external heat.
• are inclined to abuse medicines or drugs Please see “Do not use Relevtec”.
• are an athlete
This medicine may cause positive results in sports doping control tests.
Some people may become dependent on strong painkillers such as Relevtec when using them over a long period. They may have side effects when they stop using them. See section 3, “If you stop using Relevtec”.
Children and adolescents
Relevtec should not be used in children and adolescents below the age of 18 years, because no experience has so far been gained in this age group.
Other medicines and Relevtec
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
The following medicines can affect or be affected by Relevtec:
• certain medicines used to treat depression
or Parkinson's disease known as monoamine oxidase (MAO) inhibitors Do not use Relevtec if you are being treated, or have been treated in the last 2 weeks, with monoamine oxidase inhibitors.
• medicines which calm and/or induce sleep
■ anaesthetics
■ medicines to treat depression
■ medicines to treat mental or anxiety
disorders, with sedative effects
• other strong painkillers (opioids)
• certain medicines to treat bacterial infections, such as erythromycin
• certain medicines to treat fungal infections, such as ketoconazole
• certain medicines to treat HIV infections, such as ritonavir, indinavir, saquinavir
• dexamethasone, a medicine to treat many conditions including inflammation
• certain medicines to treat epilepsy or certain pain conditions, such as carbamazepine or phenytoin
• rifampicin, a medicine to treat tuberculosis or certain other infections
Relevtec with food, drink and alcohol
Avoid alcohol and products containing grapefruit while using Relevtec as they may intensify effects of buprenorphine.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
• pregnancy
There is not sufficient experience regarding use of Relevtec in pregnant women. Therefore, Relevtec transdermal patches should not be used if you are pregnant or if you could become pregnant during treatment.
A SANDOZ
• breast-feeding
Buprenorphine, the active substance contained in the transdermal patch, inhibits milk formation and passes into breast milk. Therefore, you should not use Relevtec if you are breast-feeding.
Driving and using machines
Relevtec may influence your ability to drive or use machines particularly when
• treatment starts
• switching from another preparation
• dose is changed
• you also use other medicines that act on the brain
• you drink alcohol
• treatment ends
Do not drive or use machines:
• if you feel dizzy, drowsy, experience blurred or double vision, or other effects that affect your reactions
• for at least 24 hours after the transdermal patch has been removed
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
- The medicine has been prescribed to treat a medical or dental problem and
- You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
- It was not affecting your ability to drive safely
Details regarding a new driving offence concerning driving after drugs have been taken in the UK may be found here: https://www.gov.uk/drug-driving-law.
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Consult your doctor or pharmacist if you are unsure about anything.
3 How to use Relevtec
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Your doctor chooses the appropriate strength of Relevtec for you and decides upon any changes to this, where necessary. The lowest possible dose providing adequate pain relief will be given.
The recommended dose is:
• one transdermal patch every 3 to 4 days
(maximum 96 hours)
For convenience of use you can change your transdermal patch twice weekly - always on the same days - such as always on Monday mornings and Thursday evenings. Please make a note on the outer pack to help you remember when to change your patch. Should your doctor advise you to use any additional painkiller, follow the doctor's instructions strictly. Otherwise you will not benefit fully from treatment with Relevtec.
This dose applies to all adults regardless of age, including patients with kidney diseases. Patients with liver disease require close monitoring by the doctor.
Contact your doctor or pharmacist if you think that the effect of Relevtec is too weak or too strong.
Method of administration
Transdermal use.
After application, the active substance passes through the skin into the blood.
1. Before using a transdermal patch
• Choose an area of skin on your upper body that is flat, clean and hairless. Preferably, use the chest below the collar-bone, or the upper part of the back. See pictures 1a) and 1b).
Ask for assistance if you cannot apply the transdermal patch yourself.
1a) 1b)
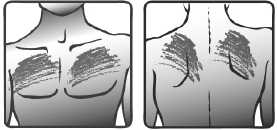
Chest Back
• If the chosen area has hair, cut it off with a pair of scissors. Do not shave it off.
• Avoid skin which is red, irritated or has any other blemishes, for instance large scars.
• The area of skin you choose must be dry and clean. If necessary, wash it with cold or lukewarm water. Do not use soap or other detergents. After a hot bath or shower, wait until your skin is completely dry and cool.
Do not apply lotion, cream or ointment to the chosen area. This might prevent your transdermal patch from sticking properly.
Sticking on the transdermal patch
Each patch is marked with “Buprenorphinum
35 pg/h”, “Buprenorphinum 52.5 pg/h” or
“Buprenorphinum 70 pg/h”.
2. Just before use, tear open the sachet where indicated, as seen in the picture. Remove the transdermal patch.
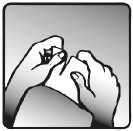
Continued on next page >>
3. The sticky side of the patch is protected with a transparent foil. Carefully peel off half the foil. Try not to touch the sticky part of the patch.

4. Stick the patch onto the area of skin you have chosen in step 1 and remove the remaining foil.
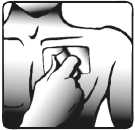
5. Press the patch against your skin with the palm of your hand. Ensure that the whole patch is in contact with your skin, especially the edges.
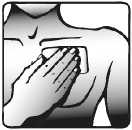
Wearing the transdermal patch
Provided that you have applied the patch correctly, there is little risk of it coming off. You may shower, bathe or swim while wearing it. However, do not expose the patch to extreme heat, such as sauna, infrared lamp, electric blanket or hot water bottle.
In the unlikely event that your patch falls off before it needs changing, do not reuse it. Stick a new one on straight away.
Changing the transdermal patch
Take the old transdermal patch off and fold it in half with the sticky side inwards. Dispose of the used patch carefully.
Always use a new appropriate site for a new patch, following steps 1 to 8 above. Wait at least one week before using the same site again.
Duration of use
This will be decided by your doctor. See also under “If you stop using Relevtec”.
If you use more Relevtec than you should
Remove the excess patches and consult a doctor immediately.
Signs of overdose are:
• drowsiness
• nausea, vomiting
• pinpoint pupils
• reduced breathing
• blood circulation collapse
If you forget to use Relevtec
Stick a new transdermal patch on as soon as you remember and make a note of the day. Amend your patch change routine. For example, if you usually change your patch on Mondays and Thursdays, but you forget and don't renew your patch until Wednesday. In this case, change your patches on Wednesdays and Saturdays from then on. If you are very late changing your transdermal patch, pain may return. Contact your doctor if this occurs.
Never apply extra patches to make up for a forgotten application.
If you stop using Relevtec
If you interrupt or finish treatment too soon, pain may return and you may feel unwell. Contact your doctor who will decide upon the necessary steps should you wish to stop treatment.
The risk of side effects after ending Relevtec transdermal patches use is very low. Consult your doctor if you feel excited, anxious, nervous, shaky, overactive, or have sleeping difficulties or digestion problems.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4 Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious allergic reactions (very rare, may affect up to 1 in 10,000 people)
Remove the transdermal patch and contact your doctor immediately if you develop any allergic reaction symptoms such as:
• swelling of the face, lips, mouth, neck, eyes or throat
• wheezing or difficulty in swallowing or breathing
• skin rash, redness or itching covering most of the body
• yellowing of the skin and eyes
• collapse, feeling faint
In some cases delayed allergic reactions occur with signs of inflammation. In such a case, remove the transdermal patch and contact your doctor.
Other side effects can occur with the following frequencies:
Very common, may affect more than 1 in 10 people
• nausea
• skin reddening, itching
Common, may affect up to 1 in 10 people
• dizziness
• headache
• shortness of breath
• vomiting, constipation
• skin changes
• sweating
• tissue swelling caused by excess fluid
• tiredness
Uncommon, may affect up to 1 in 100 people
• confusion
• sleep disorder, restlessness
• sedation, sleepiness
• blood circulation disorders such as low blood pressure and, rarely, fainting
• dry mouth
• rash
• difficulties in passing urine, urine retention
• weariness
Rare, may affect up to 1 in 1,000 people
• loss of appetite
• hallucinations, anxiety, nightmares
• decreased sexual drive
• reduced concentration
• speech or balance disorder
• drowsiness
• abnormal skin sensation such as numbness, prickling, tingling or burning
• visual disturbance such as blurred vision
• swelling of the eyelid
• hot flushes
• breathing difficulties
• heartburn
• nettle rash
• decreased erection
• withdrawal symptoms
• administration site reactions
Very rare, may affect up to 1 in 10,000 people
• dependence on the medicine
• mood swings
• muscle twitching
• taste disturbance
• pinpoint pupils
• ear or chest pain
• breathing faster than normal
• hiccups, retching
• pustules, small blisters
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
5 How to store Relevtec
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the sachet. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
Do not throw away any medicines via wastewater or household waste. Used transdermal patches should be folded with the adhesive surfaces inwards. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6 Contents of the pack and other information
What Relevtec contains
The active substance is buprenorphine.
Relevtec 35 microgram/hour transdermal patch: Each transdermal patch contains 20 mg of buprenorphine in a patch size of 25 cm2 releasing 35 micrograms of buprenorphine per hour.
Relevtec 52.5 microgram/hour transdermal patch: Each transdermal patch contains 30 mg of buprenorphine in a patch size of 37.5 cm2 releasing 52.5 micrograms of buprenorphine per hour.
Relevtec 70 microgram/hour transdermal patch: Each transdermal patch contains 40 mg of buprenorphine in a patch size of 50 cm2 releasing 70 micrograms of buprenorphine per hour.
The other ingredients are:
Release liner (to be removed before applying the patch): poly(ethylene terephthalate) foil, siliconized
Adhesive matrix (containing buprenorphine):
levulinic acid, oleyl oleate, povidone K90,
poly[butylacrylate-co-(2-ethylhexyl)acrylate-co-
methylmethacrylate-co-N-tert-octylacrylamid]
(32:32:15:20), poly[acrylic acid-co-butylacrylate-
co-(2-ethylhexyl)acrylate-co-vinylacetate]
(5:15:75:5)
Separating film (between the adhesive matrices with and without buprenorphine): poly(ethylene terephthalate) foil
Adhesive matrix (without buprenorphine): acrylate adhesive
Backing layer (printed): polyurethane backing foil, printing ink
What Relevtec looks like and contents of the pack
Relevtec is a pale yellowish-brown, rectangular transdermal patch with rounded edges, containing the following imprint:
Buprenorphinum 35 pg/h Buprenorphinum 52.5 pg/h Buprenorphinum 70 pg/h
Each transdermal patch is individually packed in a child resistant sachet.
Carton containing 2, 3, 4, 5, 6, 8, 9, 10, 11, 12,
16, 18, 19, 20, 21 or 24 transdermal patches.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder Sandoz Limited Frimley Business Park,
Frimley,
Camberley,
Surrey,
GU16 7SR,
UK
Manufacturer Hexal AG IndustriestraRe 25,
83607 Holzkirchen,
Germany
This leaflet was last revised in 06/2016
00000000
SZ00000LT000
draft: 44068782, 44068783, 44068784 laetus code: 0000 mat.no.: 00000000
Artwork Proof Box
Ref: Licence application
|
Proof no. 001.0 |
Date prepared: 17/05/2016 |
Font size: 9pt | |
|
Colours: |
Fonts: | ||
|
| Black |
□ |
Helvetica | |
|
□ |
□ |
Dimensions: 165 x 620 mm