Reminyl 4 Mg/Ml Oral Solution
Out of date information, search another2487
13.01.14[3]
PATIENT INFORMATION LEAFLET
Reminyl® 4 mg/ml Oral Solution
(galantamine hydrobromide)
Read all of this leaflet carefully before you start taking this medicine
• Keep this leaflet. You may need to read it again.
• If you are a carer and will be giving Reminyl to the person you look after, it is also important that you read this leaflet on their behalf.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine will be referred to as Reminyl throughout this leaflet.
In this leaflet:
1. What Reminyl is and what it is used for
2. Before you take Reminyl
3. How to take Reminyl
4. Possible side effects
5. How to store Reminyl
6. Further information
1. What Reminyl is and what it is used for
Reminyl is an antidementia medicine used to treat the symptoms of mild to moderately severe dementia of the Alzheimer type, a disease that alters brain function.
The symptoms of Alzheimer's disease include increasing memory loss, confusion and behavioural changes. As a result, it becomes more and more difficult to carry out normal daily activities.
These symptoms are believed to be due to a lack of acetylcholine, a substance responsible for sending messages between brain cells. Reminyl increases the amount of acetylcholine in the brain and so could improve the symptoms of the disease.
2. Before you take Reminyl Do not take Reminyl
• If you are allergic (hypersensitive) to galantamine or to any of the other ingredients listed in section 6 of this leaflet
• If you have severe liver and/or severe kidney disease
Take special care with Reminyl
Reminyl should be used in Alzheimer's disease and not other forms of memory loss or confusion.
Medicines are not always suitable for everyone. Your doctor needs to know before you take Reminyl if you suffer from or have suffered in the past from any of the following conditions:
• liver or kidney problems
• a heart disorder (e.g. angina, heart attack, heart failure, slow or irregular pulse)
• electrolyte disturbances (e.g. decreased/increased blood potassium levels)
• peptic (stomach) ulcer disease
• acute abdominal pain
• a disorder of the nervous system (like epilepsy or Parkinson's disease)
• a respiratory disease or infection that interferes with breathing (like asthma, obstructive pulmonary disease, or pneumonia)
• if you recently had an operation on the gut or bladder
• if you have difficulties passing urine.
If you need an operation which requires a general anaesthetic, you should inform the doctor that you are taking Reminyl.
Your doctor will then decide whether treatment with Reminyl is suitable for you or if the dose needs to be changed.
Taking other medicines
You should always tell the doctor, nurse or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription.
Reminyl should not be used with medicines that work in a similar way, these include:
• donepezil or rivastigmine (for Alzheimer's disease)
• ambenonium, neostigmine or pyridostigmine (for severe muscular weakness)
• pilocarpine (for dry mouth or dry eyes) if taken by mouth.
Some medicines can affect the way Reminyl works, or Reminyl itself can reduce the effectiveness of other medicines taken at the same time.
These include:
• paroxetine or fluoxetine (antidepressants)
• quinidine (used for heart rhythm problems)
• ketoconazole (antifungal)
• erythromycin (antibiotic)
• ritonavir (antiviral - HIV protease inhibitor).
Your doctor may prescribe a smaller dose of Reminyl if you are also taking any of the medicines listed above.
Some medicines can increase the number of side effects caused by Reminyl, these include:
• non-steroidal anti-inflammatory painkillers (e.g. ibuprofen) which can increase the risk of ulcers
• medicines taken for heart disorders or high blood pressure (e.g. digoxin, amiodarone, atropine, beta-blockers, or calcium channel blocking agents). If you take medicines for an irregular heart-beat, your doctor may consider an electrocardiogram (ECG).
If you need an operation which requires a general anaesthetic, you should inform the doctor that you are taking Reminyl.
If you have any questions, speak to your doctor or pharmacist for advice.
Taking Reminyl with food and drink
Reminyl should be taken with food if possible.
Drink plenty of liquids during your treatment with Reminyl, to keep yourself hydrated. See section 3 of this leaflet for full details about how to take this medicine.
Pregnancy and breast-feeding
Before taking Reminyl, speak to your doctor for advice if you are pregnant, think you could be pregnant, or you are planning a pregnancy.
You should not breastfeed while you are taking Reminyl.
Driving and using machines
Reminyl may cause dizziness or drowsiness, especially during the first few weeks of treatment. If you experience these symptoms, do not drive or use any tools or machinery.
Important information about some of the ingredients of Reminyl
This medicine contains methyl and propyl parahydroxybenzoates that can sometimes cause allergic reactions, which may possibly be delayed.
3. How to take Reminyl
Always take Reminyl exactly as your doctor has told you. You should check with your doctor if you have any questions.
How to take Reminyl oral solution
Reminyl oral solution should be taken twice daily, in the morning and evening, with water or other liquids, and preferably with food.
Reminyl is started at a low dose. Your doctor may then tell you to slowly increase the dose (strength) of Reminyl that you take, to find the most suitable dose for you.
1. The treatment is started at 4 mg (1 millilitre of solution) taken twice daily. After 4 weeks of treatment, the dose is increased.
2. You would then take 8 mg (2 millilitres of solution) twice daily. After another 4 weeks of treatment at the earliest, your doctor may decide to increase the dose again.
3. You would then take 12 mg (3 millilitres of solution) twice daily.
Your doctor will explain what dose to start with and when the dose should be increased. If you feel that the effect of Reminyl is too strong or too weak, talk to your doctor or pharmacist.
Your doctor will need to see you regularly to check that this medicine is working for you and to discuss how you are feeling. Your doctor will also check your weight regularly while you are taking Reminyl.
Liver or kidney disease
• If you have mild liver disease or mild to moderate kidney disease, the above dosing instructions are followed.
• If you have moderate liver disease, treatment is started with 4 mg oral solution once daily in the morning. After one week, begin taking the 4 mg oral solution twice daily for at least 4 weeks. Do not take more than 8 mg twice daily.
• If you have severe liver and/or kidney disease, do not take Reminyl.
The solution comes with a pipette which you should use to take the exact amount needed from the bottle.
Directions for opening the bottle and using the pipette
Fig. 1
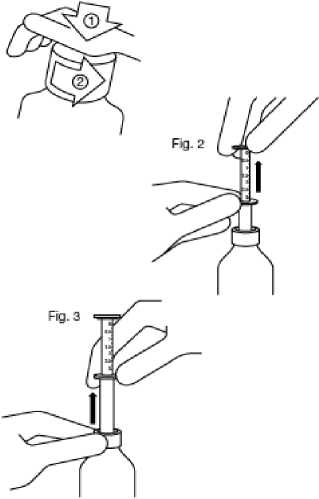
Fig. 1 : The bottle comes with a child-resistant cap, and should be opened as follows:
- Push the plastic screw cap down while turning it counter clockwise.
- Remove the unscrewed cap.
Fig. 2 : Insert the pipette into the bottle.
While holding the bottom ring, pull the top ring up to the mark corresponding to the number of millilitres you want to give.
Fig. 3: Holding the bottom ring, remove the entire pipette from the bottle. Empty the pipette into any non- alcoholic drink by sliding the upper ring down and drink it immediately.
Close the bottle.
Rinse the pipette with some water.
If you take more Reminyl than you should
If you take too much Reminyl, contact a doctor or hospital straight away. Take along any remaining solution and the packaging with you. Signs or symptoms of overdose may include, among others: severe nausea, vomiting, muscle weakness, slow heart beat, seizures and loss of consciousness.
If you forget to take Reminyl
If you forget to take one dose, miss out the forgotten dose completely and take the next dose at the normal time.
Do not take a double dose to make up for a forgotten dose.
If you forget to take more than one dose, you should contact your doctor.
If you stop taking Reminyl
You should consult your doctor before you stop taking Reminyl. It is important to continue taking this medicine to treat your condition.
Children
Reminyl is not recommended for children.
4. Possible side effects
Like all medicines, Reminyl can cause side effects, although not everybody gets them. Some of these effects may be due to the disease itself.
Stop taking your medicine and see a doctor immediately if you
experience;
• Heart problems including changes in heart beat (slow or irregular)
• Palpitations (pounding heart beat)
• Conditions like blackout
• An allergic reaction. The signs may include a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue
Side effects include:
Very Common: affects more than one user in 10.
• Feeling sick and/or vomiting. If these undesired effects occur, they are mainly experienced early on in the treatment or when the dose is increased. They tend to disappear gradually as the body gets used to the treatment and generally will not last for more than a few days. If you have these effects, your doctor may recommend that you drink more liquids and, if necessary, may prescribe a medicine to stop you being sick.
Common: affects 1 to 10 users in 100.
• Weight loss
• Loss of appetite
• Decreased appetite
• Slow heart beat
• Feeling faint
• Dizziness
• Trembling
• Headache
• Drowsiness
• Abnormally tired
• Stomach pain or discomfort
• Diarrhoea
• Indigestion
• Increased sweating
• Muscle spasms
• Falling
• High blood pressure
• Feeling weak
• General feeling of discomfort
• Seeing, feeling, or hearing things that are not real (hallucinations)
• Feeling sad (depression).
Uncommon: affects 1 to 10 users in 1000
• Increased liver enzymes in the blood (laboratory test result that tells how well your liver is working)
• Possible skipped heart beat
• Disturbance in the mechanism of conducting impulses in the heart
• Sensation of abnormal heart beats (palpitations)
• Tingling, pricking, or numbness of the skin
• Change in the sense of taste
• Excessive sleepiness
• Fit (Seizures)
• Blurred vision
• Ringing or buzzing in the ears (tinnitus)
• Feeling the need to vomit
• Muscle weakness
• Excessive water loss in the body
• Low blood pressure
• Reddening of the face
• Allergic reaction
Rare side effects: affects 1 to 10 users in 10000.
• Inflammation of the liver (hepatitis).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Reminyl
Keep out of the sight and reach of children.
Do not use Reminyl after the expiry date printed on the packaging.
The expiry date refers to the last day of that month.
Do not freeze.
Reminyl should not be used for longer than 3 months after the bottle has first been opened.
If the solution has become discoloured or shown any other signs of deterioration please tell your doctor or pharmacist before taking your medicine.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information What Reminyl contains
It contains the active ingredient galantamine hydrobromide.
Each 1 ml of oral solution contains 4 mg galantamine (as hydrobromide). Reminyl also contains the inactive ingredients methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), sodium saccharin, sodium hydroxide, purified water.
What Reminyl looks like and contents of the pack
Reminyl is available as clear colourless solution in a 100 ml amber glass bottle with child-proof cap and a measuring pipette.
Manufacturer and Product Licence Holder
Manufactured by Janssen Pharmaceutica N.V., Turnhoutseweg 30, B-2340 Beerse, Belgium. Procured from within the EU by Product Licence holder Tenolol Ltd, 5 Sandridge Close, Harrow, Middlesex HA1 1XD. Repackaged by Servipharm Ltd.
POM PL No: 30900/2487
Leaflet revision and issue date (Ref) 13.01.14[3]
Reminyl is a trademark of Shire Pharmaceutical Development Ltd.
2487
13.01.14[3]
PATIENT INFORMATION LEAFLET
Galantamine 4 mg/ml Oral Solution
(galantamine hydrobromide)
Read all of this leaflet carefully before you start taking this medicine
• Keep this leaflet. You may need to read it again.
• If you are a carer and will be giving Galantamine to the person you look after, it is also important that you read this leaflet on their behalf.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine will be referred to as Galantamine throughout this leaflet.
In this leaflet:
1. What Galantamine is and what it is used for
2. Before you take Galantamine
3. How to take Galantamine
4. Possible side effects
5. How to store Galantamine
6. Further information
1. What Galantamine is and what it is used for
Galantamine is an antidementia medicine used to treat the symptoms of mild to moderately severe dementia of the Alzheimer type, a disease that alters brain function.
The symptoms of Alzheimer's disease include increasing memory loss, confusion and behavioural changes. As a result, it becomes more and more difficult to carry out normal daily activities.
These symptoms are believed to be due to a lack of acetylcholine, a substance responsible for sending messages between brain cells. Galantamine increases the amount of acetylcholine in the brain and so could improve the symptoms of the disease.
2. Before you take Galantamine Do not take Galantamine
• If you are allergic (hypersensitive) to galantamine or to any of the other ingredients listed in section 6 of this leaflet
• If you have severe liver and/or severe kidney disease
Take special care with Galantamine
Galantamine should be used in Alzheimer's disease and not other forms of memory loss or confusion.
Medicines are not always suitable for everyone. Your doctor needs to know before you take Galantamine if you suffer from or have suffered in the past from any of the following conditions:
• liver or kidney problems
• a heart disorder (e.g. angina, heart attack, heart failure, slow or irregular pulse)
• electrolyte disturbances (e.g. decreased/increased blood potassium levels)
• peptic (stomach) ulcer disease
• acute abdominal pain
• a disorder of the nervous system (like epilepsy or Parkinson's disease)
a respiratory disease or infection that interferes with breathing (like asthma, obstructive pulmonary disease, or pneumonia) if you recently had an operation on the gut or bladder if you have difficulties passing urine.
If you need an operation which requires a general anaesthetic, you should inform the doctor that you are taking Galantamine.
Your doctor will then decide whether treatment with Galantamine is suitable for you or if the dose needs to be changed.
Taking other medicines
You should always tell the doctor, nurse or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription.
Galantamine should not be used with medicines that work in a similar way, these include:
• donepezil or rivastigmine (for Alzheimer's disease)
• ambenonium, neostigmine or pyridostigmine (for severe muscular weakness)
• pilocarpine (for dry mouth or dry eyes) if taken by mouth.
Some medicines can affect the way Galantamine works, or Galantamine itself can reduce the effectiveness of other medicines taken at the same time. These include:
• paroxetine or fluoxetine (antidepressants)
• quinidine (used for heart rhythm problems)
• ketoconazole (antifungal)
• erythromycin (antibiotic)
• ritonavir (antiviral - HIV protease inhibitor).
Your doctor may prescribe a smaller dose of Galantamine if you are also taking any of the medicines listed above.
Some medicines can increase the number of side effects caused by Galantamine, these include:
• non-steroidal anti-inflammatory painkillers (e.g. ibuprofen) which can increase the risk of ulcers
• medicines taken for heart disorders or high blood pressure (e.g. digoxin, amiodarone, atropine, beta-blockers, or calcium channel blocking agents). If you take medicines for an irregular heart-beat, your doctor may consider an electrocardiogram (ECG).
If you need an operation which requires a general anaesthetic, you should inform the doctor that you are taking Galantamine.
If you have any questions, speak to your doctor or pharmacist for advice.
Taking Galantamine with food and drink
Galantamine should be taken with food if possible.
Drink plenty of liquids during your treatment with Galantamine, to keep yourself hydrated. See section 3 of this leaflet for full details about how to take this medicine.
Pregnancy and breast-feeding
Before taking Galantamine, speak to your doctor for advice if you are pregnant, think you could be pregnant, or you are planning a pregnancy. You should not breastfeed while you are taking Galantamine.
Driving and using machines
Galantamine may cause dizziness or drowsiness, especially during the first few weeks of treatment. If you experience these symptoms, do not drive or use any tools or machinery.
Important information about some of the ingredients of Galantamine
This medicine contains methyl and propyl parahydroxybenzoates that can sometimes cause allergic reactions, which may possibly be delayed.
3. How to take Galantamine
Always take Galantamine exactly as your doctor has told you. You should check with your doctor if you have any questions.
How to take Galantamine oral solution
Galantamine oral solution should be taken twice daily, in the morning and evening, with water or other liquids, and preferably with food.
Galantamine is started at a low dose. Your doctor may then tell you to slowly increase the dose (strength) of Galantamine that you take, to find the most suitable dose for you.
1. The treatment is started at 4 mg (1 millilitre of solution) taken twice daily. After 4 weeks of treatment, the dose is increased.
2. You would then take 8 mg (2 millilitres of solution) twice daily. After another 4 weeks of treatment at the earliest, your doctor may decide to increase the dose again.
3. You would then take 12 mg (3 millilitres of solution) twice daily.
Your doctor will explain what dose to start with and when the dose should be increased. If you feel that the effect of Galantamine is too strong or too weak, talk to your doctor or pharmacist.
Your doctor will need to see you regularly to check that this medicine is working for you and to discuss how you are feeling. Your doctor will also check your weight regularly while you are taking Galantamine.
Liver or kidney disease
• If you have mild liver disease or mild to moderate kidney disease, the above dosing instructions are followed.
• If you have moderate liver disease, treatment is started with 4 mg oral solution once daily in the morning. After one week, begin taking the 4 mg oral solution twice daily for at least 4 weeks. Do not take more than 8 mg twice daily.
• If you have severe liver and/or kidney disease, do not take Galantamine.
The solution comes with a pipette which you should use to take the exact amount needed from the bottle.
Directions for opening the bottle and using the pipette
Fig. 1
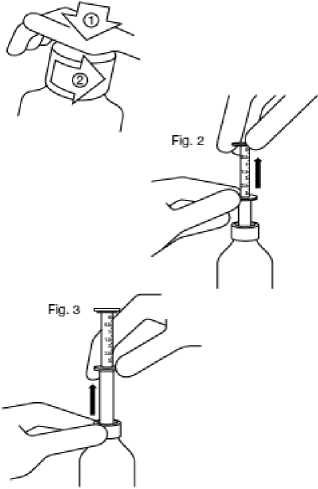
Fig. 1 : The bottle comes with a child-resistant cap, and should be opened as follows:
- Push the plastic screw cap down while turning it counter clockwise.
- Remove the unscrewed cap.
Fig. 2 : Insert the pipette into the bottle.
While holding the bottom ring, pull the top ring up to the mark corresponding to the number of millilitres you want to give.
Fig. 3: Holding the bottom ring, remove the entire pipette from the bottle. Empty the pipette into any non- alcoholic drink by sliding the upper ring down and drink it immediately.
Close the bottle.
Rinse the pipette with some water.
If you take more Galantamine than you should
If you take too much Galantamine, contact a doctor or hospital straight away. Take along any remaining solution and the packaging with you. Signs or symptoms of overdose may include, among others: severe nausea, vomiting, muscle weakness, slow heart beat, seizures and loss of consciousness.
If you forget to take Galantamine
If you forget to take one dose, miss out the forgotten dose completely and take the next dose at the normal time.
Do not take a double dose to make up for a forgotten dose.
If you forget to take more than one dose, you should contact your doctor.
If you stop taking Galantamine
You should consult your doctor before you stop taking Galantamine. It is important to continue taking this medicine to treat your condition.
Children
Galantamine is not recommended for children.
4. Possible side effects
Like all medicines, Galantamine can cause side effects, although not everybody gets them. Some of these effects may be due to the disease itself.
Stop taking your medicine and see a doctor immediately if you
experience;
• Heart problems including changes in heart beat (slow or irregular)
• Palpitations (pounding heart beat)
• Conditions like blackout
• An allergic reaction. The signs may include a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue
Side effects include:
Very Common: affects more than one user in 10.
• Feeling sick and/or vomiting. If these undesired effects occur, they are mainly experienced early on in the treatment or when the dose is increased. They tend to disappear gradually as the body gets used to the treatment and generally will not last for more than a few days. If you have these effects, your doctor may recommend that you drink more liquids and, if necessary, may prescribe a medicine to stop you being sick.
Common: affects 1 to 10 users in 100.
• Weight loss
• Loss of appetite
• Decreased appetite
• Slow heart beat
• Feeling faint
• Dizziness
• Trembling
• Headache
• Drowsiness
• Abnormally tired
• Stomach pain or discomfort
• Diarrhoea
• Indigestion
• Increased sweating
• Muscle spasms
• Falling
• High blood pressure
• Feeling weak
• General feeling of discomfort
• Seeing, feeling, or hearing things that are not real (hallucinations)
• Feeling sad (depression).
Uncommon: affects 1 to 10 users in 1000
• Increased liver enzymes in the blood (laboratory test result that tells how well your liver is working)
• Possible skipped heart beat
• Disturbance in the mechanism of conducting impulses in the heart
• Sensation of abnormal heart beats (palpitations)
• Tingling, pricking, or numbness of the skin
• Change in the sense of taste
• Excessive sleepiness
• Fit (Seizures)
• Blurred vision
• Ringing or buzzing in the ears (tinnitus)
• Feeling the need to vomit
• Muscle weakness
• Excessive water loss in the body
• Low blood pressure
• Reddening of the face
• Allergic reaction
Rare side effects: affects 1 to 10 users in 10000.
• Inflammation of the liver (hepatitis).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Galantamine
Keep out of the sight and reach of children.
Do not use Galantamine after the expiry date printed on the packaging. The expiry date refers to the last day of that month.
Do not freeze.
Galantamine should not be used for longer than 3 months after the bottle has first been opened.
If the solution has become discoloured or shown any other signs of deterioration please tell your doctor or pharmacist before taking your medicine.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information What Galantamine contains
It contains the active ingredient galantamine hydrobromide.
Each 1 ml of oral solution contains 4 mg of galantamine (as hydrobromide).
Galantamine also contains the inactive ingredients
methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate
(E216), sodium saccharin, sodium hydroxide, purified water
What Galantamine looks like and contents of the pack
Galantamine is available as clear colourless solution in a 100 ml amber glass bottle with child-proof cap and a measuring pipette.
Manufacturer and Product Licence Holder
Manufactured by Janssen Pharmaceutica N.V., Turnhoutseweg 30, B-2340 Beerse, Belgium. Procured from within the EU by Product Licence holder Tenolol Ltd, 5 Sandridge Close, Harrow, Middlesex HA1 1XD. Repackaged by Servipharm Ltd.
POM PL No: 30900/2487
Leaflet revision and issue date (Ref) 13.01.14[3]