Renocontin 30 Mg Prolonged-Release Tablets
10 mm
◄-H
10 mm
Package leaflet: Information for the patient
Renocontin 15 mg prolonged-release tablets Renocontin 20 mg prolonged-release tablets Renocontin 30 mg prolonged-release tablets Renocontin 40 mg prolonged-release tablets Renocontin 60 mg prolonged-release tablets Renocontin 80 mg prolonged-release tablets
Oxycodone hydrochloride
Read all of this leaflet carefully before you start
taking this medicine because it contains important
information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Renocontin is and what it is used for
2. What you need to know before you take Renocontin
3. How to take Renocontin
4. Possible side effects
5. How to store Renocontin
6. Content of the pack and other information
1. What Renocontin is and what it is used for
335 mm 335 mm
Renocontin (oxycodone hydrochloride) is a centrally acting, strong painkiller from the group of opioids. Renocontin is used to treat severe pain, which can be adequately managed only with opioid analgesics. Renocontin is indicated in adults and adolescents aged 12 years and older.
2. What you need to know before you take Renocontin
Do not take Renocontin
- if you are allergic to oxycodone hydrochloride or any of the other ingredients of this medicine (listed in section 6),
- have breathing problems, such as chronic obstructive lung disease, severe bronchial asthma or respiratory depression. Your doctor will have told you if you have any of these conditions. Symptoms may include breathlessness, coughing or breathing more slowly or weakly than expected,
- have a condition where the small bowel does not work properly (paralytic ileus),
- your stomach empties more slowly than it should (delayed gastric emptying) or you have severe pain in your abdomen,
Warnings and precautions
Talk to your doctor or pharmacist before taking Renocontin
- if you are elderly or weakened,
- if you have lung, liver or kidney problems,
- if you suffer from myxoedema (a thyroid disorder with dryness, coldness and swelling [‘puffiness’] of the skin affecting the face and limbs), impaired function of the thyroid gland,
- if you have poor adrenal gland function (your adrenal gland is not working properly which may cause symptoms including weakness, weight loss, dizziness, feeling or being sick) e.g. Addison’s disease,
- if you have an enlarged prostate gland, which causes difficulty in passing urine (prostatic hypertrophy),
- have previously suffered from withdrawal symptoms such as agitation, anxiety, shaking or sweating upon stopping taking alcohol or drugs,
- are or have ever been addicted to alcohol or drugs or have a known opioid dependence,
- if you have inflammatory bowel disease,
- if you have inflammation of the pancreas which causes
^ severe pain in the abdomen and back (pancreatitis),
- if you have head injury, severe headache or feel sick as this may indicate that the pressure in your skull is increased,
- if you have disturbances of your blood circulation such as fluid retention (oedema), blood loss (haemorrhage), blood clots, etc,
- if you have problems with the gall bladder, bile duct or the ureter,
- if you have epilepsy or have tendency to suffer seizures, fits or convulsions,
- if you take MAO inhibitors (a medicine for the treatment of depression),
Talk to your doctor if any of these apply to you or if any of these conditions applied to you in the past. If you are going to have an operation, please tell the doctor at the hospital that you are taking these tablets.
Dependence and tolerance
Renocontin has a primary dependence potential.
When used for a long time tolerance to the effects and progressively higher doses may be required to maintain pain control.
Chronic use of Renocontin may lead to physical dependence and a withdrawal syndrome may occur if you stop using the product suddenly. When a patient no longer requires therapy with oxycodone hydrochloride, it may advisable to decrease the dose gradually to prevent symptoms of withdrawal.
When used as directed in patients suffering from chronic pain the risk of developing physical or psychological dependence is markedly reduced and needs to be weighed against the potential benefit. Please discuss this with your doctor.
Renocontin are for oral use only. In case of abusive injection (injection in a vein) the tablet excipients (especially talc) may lead to destruction (necrosis) of the local tissue, change of lung tissue (granulomas of the lung) or other serious, potentially fatal events.
This medicine should be avoided in patients with a history of or present alcohol and drug abuse.
Anti-doping warning
Athletes should be aware that this medicine may cause a positive reaction to “anti-doping tests”. Use of Renocontin as a doping agent may become a health hazard.
Children and adolescents
Oxycodone has not been investigated in children under 12 years. Safety and efficacy have not been established therefore use in children under 12 years of age is not recommended.
Other medicines and Renocontin
Tell your doctor or pharmacist if you are taking, have recently taken any other medicines, including medicines obtained without a prescription. If you take these tablets with some other medicines, the effect of these tablets or the other medicine may be changed.
The tablets must not be used together with a monoamine oxidase inhibitor, or if you have taken this type of medicine in the last two weeks (see section 2 ‘Do not take...’).
Tell your doctor or pharmacist if you are taking:
• medicines to help you sleep or stay calm (for example tranquillisers, hypnotics or sedatives)
• medicines to treat depression (for example paroxetine)
• medicines to treat psychiatric or mental disorders (such as phenothiazines or neuroleptic drugs)
• other strong analgesics (‘painkillers’)
• muscle relaxants
• medicines to treat high blood pressure
• quinidine (a medicine to treat a fast heartbeat)
• cimetidine (a medicine for stomach ulcers, indigestion or heartburn)
• medicines to treat fungal infections (such as ketoconazole, voriconazole, itraconazole, or posaconazole)
• medicines used to treat infections (such as clarithromycin, erythromycin or telithromycin)
• a specific type of medicine known as a protease inhibitor to treat HIV (examples include boceprevir, ritonavir, indinavir, nelfinavir or saquinavir)
• rifampicin to treat tuberculosis
• carbamazepine (a medicine to treat seizures, fits or convulsions and certain pain conditions)
• phenytoin (a medicine to treat seizures, fits or convulsions)
• a herbal remedy called St John’s Wort (also known as Hypericum perforatum).
Also, tell your doctor if you have recently been given an anaesthetic.
Renocontin with food, drink and alcohol
Drinking alcohol whilst taking Renocontin may make you feel more sleepy or increase the risk of serious side effects such as shallow breathing with a risk of stopping breathing, and loss of consciousness. It is recommended not to drink alcohol while you are taking Renocontin.
You should avoid drinking grapefruit juice during your treatment with Renocontin Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Pregnancy
Renocontin should not be taken in pregnancy unless clearly necessary. There are only limited data from the use of oxycodone in pregnant women.
Oxycodone crosses the placenta into the blood circulation of the baby.
Prolonged use of oxycodone during pregnancy can cause withdrawal symptoms in newborns. Use of oxycodone during delivery can cause respiratory depression in the newborn.
Breast-feeding
You should not take Renocontin when you are breastfeeding as oxycodone passes into breast milk.
Driving and using machines
Oxycodone hydrochloride may cause a number of side effects such as drowsiness which could affect your ability to drive or use machinery (see section 4 for a full list of side effects) impairs alertness and reactivity to such an extent that the ability to drive and operate machinery is affected or ceases altogether. To look at the possible side effects affecting the motor skills and concentration (see section 4).
With stable therapy, a general ban on driving a vehicle may be not necessary. The treating physician must assess the individual situation. Please discuss with your doctor whether or under what conditions you can drive a vehicle. The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
- The medicine has been prescribed to treat a medical or dental problem and
- You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
- It was not affecting your ability to drive safely Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Renocontin contains lactose
If you have been told by your doctor that you have an intolerance to some sugars contact your doctor before taking this medicinal product.
3. How to take Renocontin
10 mm
8,5 mm
+1,5 mm bleed

Read Direction (Code centered)
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
Adults and adolescents (12 years of age and older)
The usual initial dose is 10 mg oxycodone hydrochloride in 12 hourly intervals. However, your doctor will prescribe the dose required to treat pain.
Patients who have already taken opioids can start treatment with higher dosages taking into account their experience with opioid treatment.
For the treatment of non cancer pain a daily dose of 40 mg of oxycodone hydrochloride is generally sufficient, but higher dosages may be necessary.
Patients with cancer pain usually require dosages from 80 to 120 mg of oxycodone hydrochloride which may be increased up to 400 mg in individual cases.
For those doses where this particular strength is not suitable for you, other strengths of this medicinal product are available.
Risk patients
If you have impaired kidney and/or liver function or if you have a low body weight your doctor may prescribe a lower starting dose.
Use in children and adolescents
Renocontin is not recommended in children younger than 12 years of age.
Method of administration
Swallow the prolonged-release tablet whole with a sufficient amount of liquid (^ glass of water) with or without food in the morning and in the evening following a fixed schedule (e.g. at 8 a.m. and 8 p.m.).
The tablets must be swallowed whole, not chewed, divided or crushed as this leads to rapid oxycodone release due to the damage of the prolonged release properties. The administration of chewed, divided or crushed prolonged-release tablets leads to a rapid release and absorption of a potentially fatal dose of oxycodone (see section “If you take more Renocontin than you should”).
Renocontin should not be taken with alcoholic beverages. Opening instructions:
This medicinal product is in child resistant packaging.
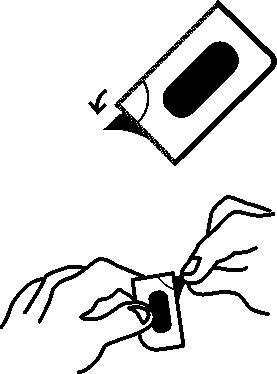
The prolonged-release tablets cannot be pressed out of the blister. Please observe the following instructions when opening the blister.
1. Pull off a single dose by tearing along the perforated line on the blister.
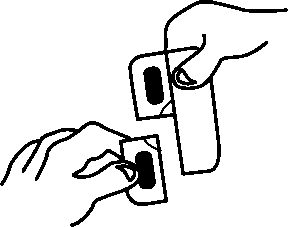
2. An unsealed area is exposed/can be reached by this; this area is at the point where the perforated lines intersect with each other.
3. At the unsealed flap, peel away the cover foil from the bottom foil.
10 mm
Stanzkontur_200 x 690 mm Laetus.indd 1
25.01.16 13:49
10 mm <-►
Your doctor will decide your daily dose and when this should be taken, depending on the level of pain you are being treated for. Your dose may be changed during treatment if your doctor thinks it is necessary. You must not change the dose yourself.
Some patients who take this medicine regularly each day, sometimes need additional rapid acting pain relief to control breakthrough pain. You should not use additional doses of Renocontin to control breakthrough pain.
Your doctor will regularly monitor your treatment to make sure that your pain is adequately controlled, to treat any side effects that you may experience and to decide if your treatment needs to be continued.
If you take more Renocontin than you should If you have taken more of this medicine than prescribed by your doctor you should seek immediate medical advice. The following symptoms may occur: constricted pupils (miosis), breathing difficulties (respiratory depression), drowsiness, skeletal muscle flaccidity and drop in blood pressure. In severe cases you may experience problems with your heart and circulation, have severe difficulty with breathing and lose consciousness. Taking high doses of strong opioids like oxycodone can be fatal. You should not drive yourself to hospital if you have taken too many tablets.
If you forget to take Renocontin
If you miss a dose, your pain may not be controlled. If there are at least 8hrs until your next due dose, you can take the forgotten dose when you remember. You then continue with your medicine as scheduled.
10 mm If there is less than 8hrs until your next dose, you should
<-Kake the missed dose but make sure that you leave at least
8hrs until the next dose. You must not take this medicine more than once every eight hours. Never take a double dose to make up for a missed dose.
If you stop taking Renocontin
You must not stop taking this medicine without asking
for your doctor’s advice. When you no longer need
these tablets, your doctor will gradually lower your
dose to prevent you experiencing unpleasant withdrawal
symptoms.
If you have any further questions on how to take this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following side effects, stop taking Renocontin and contact your doctor immediately:
All medicines can cause allergic reactions, although serious allergic reactions are uncommon. Tell your doctor immediately if you get any sudden wheeziness, difficulties in breathing, swelling of the eyelids, face or lips, rash or itching especially those covering your whole body.
Depressed in breathing is the most significant risk induced by opioids and is most likely to occur in elderly or debilitated patients. As a consequence, in predisposed patients opioids can cause severe drops in blood pressure.
Apart from this oxycodone can cause constricted pupils, bronchial spasms and spasms in smooth muscles and suppress the cough reflex.
Other possible side effects
Very common (may affect more than 1 in 10 people):
• Sedation (tiredness to drowsiness); dizziness; headache; constipation; nausea; vomiting; itching.
Common (may affect up to 1 in 10 people):
• Several psychological side effects such as changes in mood (e.g. anxiety, depression); changes in activity (mostly sedation, sometimes accompanied by lethargy, occasionally increase with nervousness and sleep disorders) and changes in performance (thought process disorder, confusion, isolated cases of speech disorders),
• feeling weak (asthenia); trembling (tremor),
• difficulty in breathing or wheezing (dyspnoea, bronchospasm),
• dry mouth, rarely accompanied by thirst and difficulty swallowing; gastrointestinal disorders such as bellyache; diarrhoea; upset stomach (dyspepsia); loss of appetite,
• skin disorders such as rash, rarely increased sensitivity to light (photosensitivity), in isolated cases itchy (urticaria) or scaly rash (exfoliative dermatitis),
• urinary disorders (frequent urination), increased sweating (hyperhidrosis),
Uncommon (may affect up to 1 in 100 people):
• A condition which causes abnormal production of a hormone reducing urination (syndrome of inappropriate antidiuretic hormone secretion),
• change in perception such as depersonalisation, hallucinations (perception of things that are not there), emotional instability, change in taste, visual disturbances, abnormally acute sense of hearing (hyperacousis); euphoria; restlessness,
• increased and decreased muscle tone involuntary muscle contractions, disturbance of memory (amnesia); fits, speech disorder; reduced sense of touch (hypaesthesia); coordination disturbances; feeling unwell; fainting; pins and needles (paraesthesia); feeling of spinning (vertigo),
• accelerated pulse; fast or irregular beating of the heart (supraventricular tachycardia, palpitations (in context of withdrawal syndrome), widening of the blood vessels (vasodilatation),
• increased coughing; inflammation of the throat (pharyngitis); runny nose; voice changes,
• oral ulcers; inflammation of the gums, inflamed mouth (stomatitis); difficult in swallowing(dysphagia), passing wind (flatulence),
• belching; a condition where the bowel does not work properly (ileus), taste disturbance, a worsening of liver function tests (seen in a blood test),
• dry skin,
• urinary retention,
• disturbances of sexual function (reduced sexual desire and impotence),
• accidental injuries; pain (e.g. chest pain); buildup of fluid in the body causing the affected tissue to become swollen (oedema); migraine; physical dependence producing symptoms and signs of withdrawal when the product is stopped suddenly, ; lack of water in the body (dehydration), hypersensitivity which may include symptoms as wheezing, chest tightness, swelling of the lips, eyes or face or rash and itching (allergic reactions), thirst, a problem with the flow of tears in the eyes (lacrimation disorder), chills,
a ringing or buzzing sound in the ears (tinnitus), drug tolerance (i.e. an increase in dose becomes necessary to achieve the desired effect).
Rare (may affect up to 1 in 1,000 people):
Swollen lymph nodes (lymphadenopathy), seizures, in particular in patients suffering from epilepsy or with a tendency to seizures, muscle spasms (involuntary contraction of the muscle), lowering of blood pressure, rarely accompanied by symptoms such as pounding or racing heartbeat, gum bleeding; increased appetite; tarry stool; tooth staining and damage,
herpes simplex (disorder of the skin and mucosa), hives (urticaria),
changes in body weight (loss or rise); cellulitis. Frequency not known (frequency cannot be estimated from the available data)
Serious allergic reaction which causes difficulty in breathing or dizziness (anaphylactic reaction), aggression,
increased sensibility to pain (hyperalgesia), tooth decay, A blockage in the flow of bile from the liver (cholestasis). This can cause itchy skin, yellow skin, very dark urine and very pale stools, colicky abdominal pain or discomfort (biliary colic), absence of menstrual bleeding (amenorrhoea).
Opioid withdrawal syndrome
As oxycodone hydrochloride has the potential to cause a drug addiction, there is a possibility to develop an opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, watery eyes (lacrimation), nasal discharge (rhinorrhea), yawning, perspiration, chills, muscle pain, dilation of the pupil and irregular heartbeat (palpitations). Other symptoms also may develop including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate or heart rate.
Counteractive measures
If you observe any of the above listed side effects your doctor usually will take appropriate measures.
The side effect constipation may be prevented by fiber enriched diet and increased drinking.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Renocontin
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the blister and the carton after “EXP”. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Content of the pack and other information
What Renocontin contains
- The active substance is oxycodone hydrochloride.
[15 mg]:
Each prolonged-release tablet contains 15 mg oxycodone hydrochloride corresponding to 13.5 mg oxycodone.
The other ingredients are:
Tablet core:
Lactose monohydrate, Ammonio Methacrylate Copolymer, Type B dispersion 30%, Povidone (K29/32), Talc, Triacetin, Stearyl alcohol, Magnesium stearate
Tablet coating:
Hypromellose, Talc, Macrogol 400, Titanium dioxide (E171), Iron oxide black (E172)
[20 mg]:
Each prolonged-release tablet contains 20 mg oxycodone hydrochloride corresponding to 17.9 mg oxycodone.
The other ingredients are:
Tablet core:
Lactose monohydrate, Ammonio Methacrylate Copolymer, Type B dispersion 30%, Povidone (K29/32), Talc, Triacetin, Stearyl alcohol, Magnesium stearate,
Tablet coating:
Hypromellose, Talc, Macrogol 400, Titanium dioxide (E171), Iron oxide red (E172),
[30 mg]:
Each prolonged-release tablet contains 30 mg oxycodone hydrochloride corresponding to 26.9 mg oxycodone.
The other ingredients are:
Tablet core:
Lactose monohydrate, Ammonio Methacrylate Copolymer, Type B dispersion 30%, Povidone (K29/32), Talc, Triacetin, Stearyl alcohol, Magnesium stearate
Tablet coating:
Hypromellose, Talc, Macrogol 400, Titanium dioxide (E171), Iron oxide brown (E172), Iron oxide black (E172)
[40 mg]:
Each prolonged-release tablet contains 40 mg oxycodone hydrochloride corresponding to 35.9 mg oxycodone.
The other ingredients are:
Tablet core:
Lactose monohydrate, Ammonio Methacrylate Copolymer, Type B dispersion 30%, Povidone (K29/32), Talc, Triacetin, Stearyl alcohol, Magnesium stearate
Tablet coating:
Hypromellose, Talc, Macrogol 400, Titanium dioxide (E171), Iron oxide red (E172), Iron oxide yellow (E172)
[60 mg]:
Each prolonged-release tablet contains 60 mg oxycodone hydrochloride corresponding to 53.8 mg oxycodone.
The other ingredients are:
Tablet core:
Lactose monohydrate, Ammonio Methacrylate Copolymer, Type B dispersion 30%, Povidone (K29/32), Talc, Triacetin, Stearyl alcohol, Magnesium stearate
Tablet coating:
Hypromellose, Talc, Macrogol 400, Titanium dioxide (E171), Iron oxide red (E172), Erythrosine (E127)
[80 mg]:
Each prolonged-release tablet contains 80 mg oxycodone hydrochloride corresponding to 71.7 mg oxycodone.
The other ingredients are:
Tablet core:
Lactose monohydrate, Ammonio Methacrylate Copolymer, Type B dispersion 30%, Povidone (K29/32), Talc, Triacetin, Stearyl alcohol, Magnesium stearate
Tablet coating:
Hypromellose, Talc, Macrogol 400, Titanium dioxide (E171), Indigo carmine (E132), Iron oxide yellow (E172)
What Renocontin looks like and contents of the pack
[Renocontin 15 mg prolonged-release tablets:]
Grey, round, biconvex, prolonged-release tablets with a diameter of 6.9 - 7.3 mm and a height of 3.5 - 4.2 mm. [Renocontin 20 mg prolonged-release tablets:]
Light pink, round, biconvex, prolonged-releasetablets with a diameter of 6.9 - 7.3 mm and a height of 3.5 - 4.2 mm. [Renocontin 30 mg prolonged-release tablets:]
Brown, round, biconvex, prolonged-release tablets with a diameter of 6.9 - 7.3 mm and a height of 3.5 - 4.2 mm. [Renocontin 40 mg prolonged-release tablets:]
Light orange to ochre, round, biconvex, prolonged-release tablets with a diameter of 6.9 - 7.3 mm and a height of 3.5 - 4.2 mm.
[Renocontin 60 mg prolonged-release tablets:]
Pink-red, round, biconvex, prolonged-release tablets with a diameter of 8.6 - 9.0 mm and a height of 5.0 - 5.6 mm. [Renocontin 80 mg prolonged-release tablets:]
Green, round, biconvex, prolonged-release tablets with a diameter of 8.6 - 9.0 mm and a height of 5.0 - 5.6 mm. Renocontin is available for 10, 14, 20, 25, 28, 30, 40, 50, 56, 60, 98 and 100 prolonged-release tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Glenmark Pharmaceuticals Europe Limited Laxmi House, 2B Draycott Avenue, Kenton, Middlesex HA3 0BU United Kingdom
Manufacturer:
PS Pharma Service GmbH Lise-Meitner-Strahe 10 40670 Meerbusch Germany
This leaflet was last revised in October 2016
00000000-0000
Stanzkontur_200 x 690 mm Laetus.indd 2
25.01.16 13:49