Rimexolone 1% Eye Drops
Ref: 0544/170216/1/F
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any of the side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Vexol 1 % Eye Drops, throughout the remainder of this leaflet it will be referred to as Vexol
What is in this leaflet
^ What Vexol is and what it is used for ^ What you need to know before you use Vexol ^ How to use Vexol ^ Possible side effects ^ How to store Vexol
Contents of the pack and other information
What Vexol is and what it is used for
Vexol belongs to a group of medicines known as corticosteroids.
It is used to prevent or treat eye inflammation following surgery of the eye and to treat inflammation of the eye surface and the front portion inside the eye (anterior segment).
It helps to relieve the symptoms of inflammation such as redness, soreness and swelling.
Pregnancy and breast-feeding
If you are pregnant or might get pregnant, or if you are breast-feeding a baby, talk to your doctor before you use Vexol.
Driving and using machines
If your vision is blurred or your sight is affected in any way following the use of Vexol, you should not drive or operate machinery until your vision is clear.
Other medicines and VEXOL Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are using more than one eye preparation,
wait 15 minutes between each application.
Important information if you wear Contact Lenses
Wearing contact lenses is not advisable during treatment of an eye inflammation as it may make your condition worse.
Do not use the drops while wearing contact lenses. Wait at least 15 minutes after use before putting your lenses back in. There is a preservative in Vexol (benzalkonium chloride) that can discolour soft contact lenses and may cause eye irritation.
^2) What you need to know before you use Vexol
Do not use Vexol...
• If you have any type of infection of the eye that is not being treated. Use of steroids may make infections worse.
• If you have a red eye that has not been seen by a doctor.
• If you are allergic to rimexolone or any of the other ingredients listed in section 6.
Ask your doctor for advice.
Vexol is not for use in CHILDREN.
Take special care...
• If you have a disorder causing a thinning of the eye tissues, such as rheumatoid arthritis, Fuch's dystrophy or following a corneal transplant. Steroids may cause further thinning and possible perforation.
• Steroids applied to the eye may delay the healing of your eye wound.
You may still be able to use Vexol, but discuss it
with your doctor first.
3 How to use Vexol The usual dose
This will depend on the reason for use.
Inflammation (red, painful eye)
Apply 1 drop in the affected eye 4 times a day or more often, if advised by your doctor.
Inflammation of the inside of the eye (Uveitis)
1st week - Apply 1 drop in the affected eye every hour while awake.
2nd week - Apply 1 drop every 2 hours while awake. 3rd week - Apply 1 drop 4 times a day.
4th week - Apply 1 drop twice daily for the first 4 days and then apply 1 drop once daily for the last 3 days.
Inflammation after eye surgery
Apply 1 drop in the affected eye 4 times a day, beginning 24 hours after your operation and continuing for the first 2 weeks afterwards.
Not for use in CHILDREN.
Remove the loose collar from the cap when the bottle is first opened.
Always use Vexol exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How to use
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
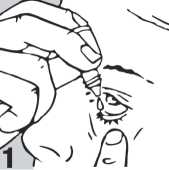
• Pull down your lower eyelid with a finger, until there is a 'pocket' between the eyelid and your eye. The drop will go in here (picture 1).
• Bring the bottle tip close to the eye. Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops.
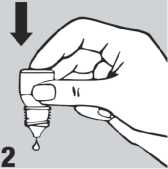
• Gently press on the base of the bottle to release one drop at a time (picture 2).
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
• If a drop misses your eye, try again.
• If you forget to use Vexol, just use it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your normal dosing schedule. Do not take a double dose to make up.
• If you use more Vexol than you should, it can be washed out with luke warm water.
• If treatment with Vexol is stopped...
A flare-up of inflammation may occur if treatment is discontinued early. Do not suddenly stop using the product without your doctor's advice. Your doctor may want to gradually reduce the amount you use to reduce the chance of unwanted effects.
Possible side effects
Like all medicines, Vexol can cause side effects, although not everybody gets them.
• You may experience some or all of the following effects in your eye(s):
Common ( affect 1 to 10 people in 100 ): Blurred vision, discharge, discomfort.
Uncommon ( affect 1 to 10 people in 1000 ): Redness of the eye or inside the eyelid, pain, itching, dry eyes, swelling, watery eyes, eye staining, irritation, serious infections, increased pressure in the eye, eye surface inflammation or other disorders.
Rare ( 1 to 10 users in 10,000 ): Swelling of the back of the eye, eyelid swelling, sensitivity to light, crusting on the eyelids or scarring or other disorders.
Not known ( cannot be estimated from the available data ): Reduced vision.
• You may also experience effects in other areas of your body including:
Uncommon ( affect 1 in 10 people in 1000 ): Headache, redness or soreness of the throat, bad or unusual taste.
Rare ( 1 in 10 users in 10,000 ): Allergy, low blood pressure, runny nose.
Not known ( cannot be estimated from the available data ): Chest pain.
If VEXOL is used for a long time this can lead to an increase in pressure inside the eye or the formation of cataracts, both of which can lead to decreased vision. It can also lead to infections, as your natural resistance to these is reduced. The risk of VEXOL induced increased pressure inside the eye may be greater in children and may occur earlier than in adults. VEXOL is not approved for use in children.
If any of the side effects get serious, or you notice any side effects not listed in this leaflet, tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you help provide more information on the safety of this medicine.
3 How to store Vexol EXPIRY DATE
Do not use this medicine after date shown on the carton label on or bottle. Only keep this medicine if your doctor tells you to. If your eye drops become discoloured or show any other signs of deterioration, consult your pharmacist (chemist) who will tell you what to do.
HOW TO STORE VEXOL
• Do not store above 30°C.
• Do not freeze.
• KEEP THIS MEDICINE OUT OF THE SIGHT AND REACH OF CHILDREN.
• Once the bottle has been opened then the eye drops must be discarded one month after first opening.
[6 Contents of the pack and other information
What Vexol contains
Vexol contains 10mg/ml of rimexolone as the active ingredient. Vexol also contains benzalkonium chloride, mannitol, carbomer, polysorbate 80, sodium chloride, disodium edetate, sodium hydroxide, hydrochloric acid, purified water.
What Vexol looks like and contents of the pack
Vexol is a white to off-white suspension in a plastic dropper bottle.
Vexol is available in bottles of 5ml of solution.
Manufacturer and Licence Holder
Vexol is manufactured by Alcon Cusi, S.A C/Camil Fabra 58, 08320 EI Masnou-Barcelona, Spain and is procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited,
Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE.
POM PL 15184/0544
Leaflet revision date: 17/02/16
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Ref: 0544/170216/1/B